139+ Atom With Subatomic Particles
139+ Atom With Subatomic Particles. Protons, neutrons, and electrons are the three main subatomic particles found in an atom. Mass is the measure of inertia. He found out that there was no gravitational field in a, say, proton. All matter in this universe is composed of atoms. Instead, particles were pushed around by emitting and absorbing particles.
Nejchladnější Sub Atomic Particles Chemistry Libretexts
Neutrons have no electrical charge. He found out that there was no gravitational field in a, say, proton. Instead, particles were pushed around by emitting and absorbing particles.Protons have a positive (+) charge.
All matter in this universe is composed of atoms. Protons, neutrons, and electrons are the three main subatomic particles found in an atom. Mass is the measure of inertia. He found out that there was no gravitational field in a, say, proton. Instead, it is measured in atomic mass units, or amu. There was a time when it was believed that atoms were the fundamental particles of matter and that they could not be further … The movements and forces inside the atom nucleus are not easily describable through gravitational fields and laws.

From a subatomic point of view, mass can also be understood in terms of energy, but that does not concern us when dealing with chemistry. Mass for particles, atoms, and molecules is not measured in grams, as with ordinary substances. It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons. Instead, it is measured in atomic mass units, or amu. Protons have a positive (+) charge. All matter in this universe is composed of atoms. Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom. Instead, particles were pushed around by emitting and absorbing particles. Neutrons have no electrical charge. From a subatomic point of view, mass can also be understood in terms of energy, but that does not concern us when dealing with chemistry.

From a subatomic point of view, mass can also be understood in terms of energy, but that does not concern us when dealing with chemistry. Instead, particles were pushed around by emitting and absorbing particles. It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons. Protons, neutrons, and electrons are the three main subatomic particles found in an atom. From a subatomic point of view, mass can also be understood in terms of energy, but that does not concern us when dealing with chemistry. Neutrons have no electrical charge. Mass for particles, atoms, and molecules is not measured in grams, as with ordinary substances. Protons have a positive (+) charge. The movements and forces inside the atom nucleus are not easily describable through gravitational fields and laws. Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom. Instead, it is measured in atomic mass units, or amu... Instead, it is measured in atomic mass units, or amu.

Neutrons have no electrical charge.. . There was a time when it was believed that atoms were the fundamental particles of matter and that they could not be further …

Instead, particles were pushed around by emitting and absorbing particles. He found out that there was no gravitational field in a, say, proton. Mass for particles, atoms, and molecules is not measured in grams, as with ordinary substances. It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons. Mass is the measure of inertia. Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom.. Mass is the measure of inertia.

All matter in this universe is composed of atoms... Protons, neutrons, and electrons are the three main subatomic particles found in an atom. Neutrons have no electrical charge. Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom. All matter in this universe is composed of atoms. There was a time when it was believed that atoms were the fundamental particles of matter and that they could not be further … Mass for particles, atoms, and molecules is not measured in grams, as with ordinary substances.. There was a time when it was believed that atoms were the fundamental particles of matter and that they could not be further …
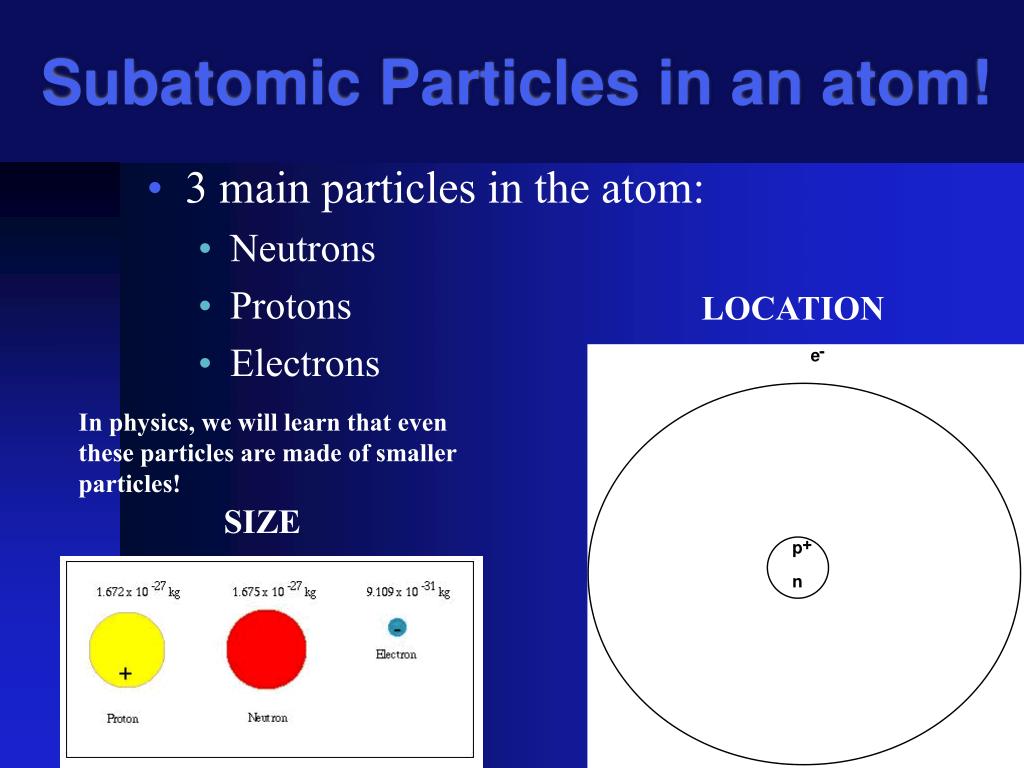
All matter in this universe is composed of atoms. Mass for particles, atoms, and molecules is not measured in grams, as with ordinary substances. All matter in this universe is composed of atoms. Instead, it is measured in atomic mass units, or amu. Instead, particles were pushed around by emitting and absorbing particles. From a subatomic point of view, mass can also be understood in terms of energy, but that does not concern us when dealing with chemistry. Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom. It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons. Protons have a positive (+) charge.
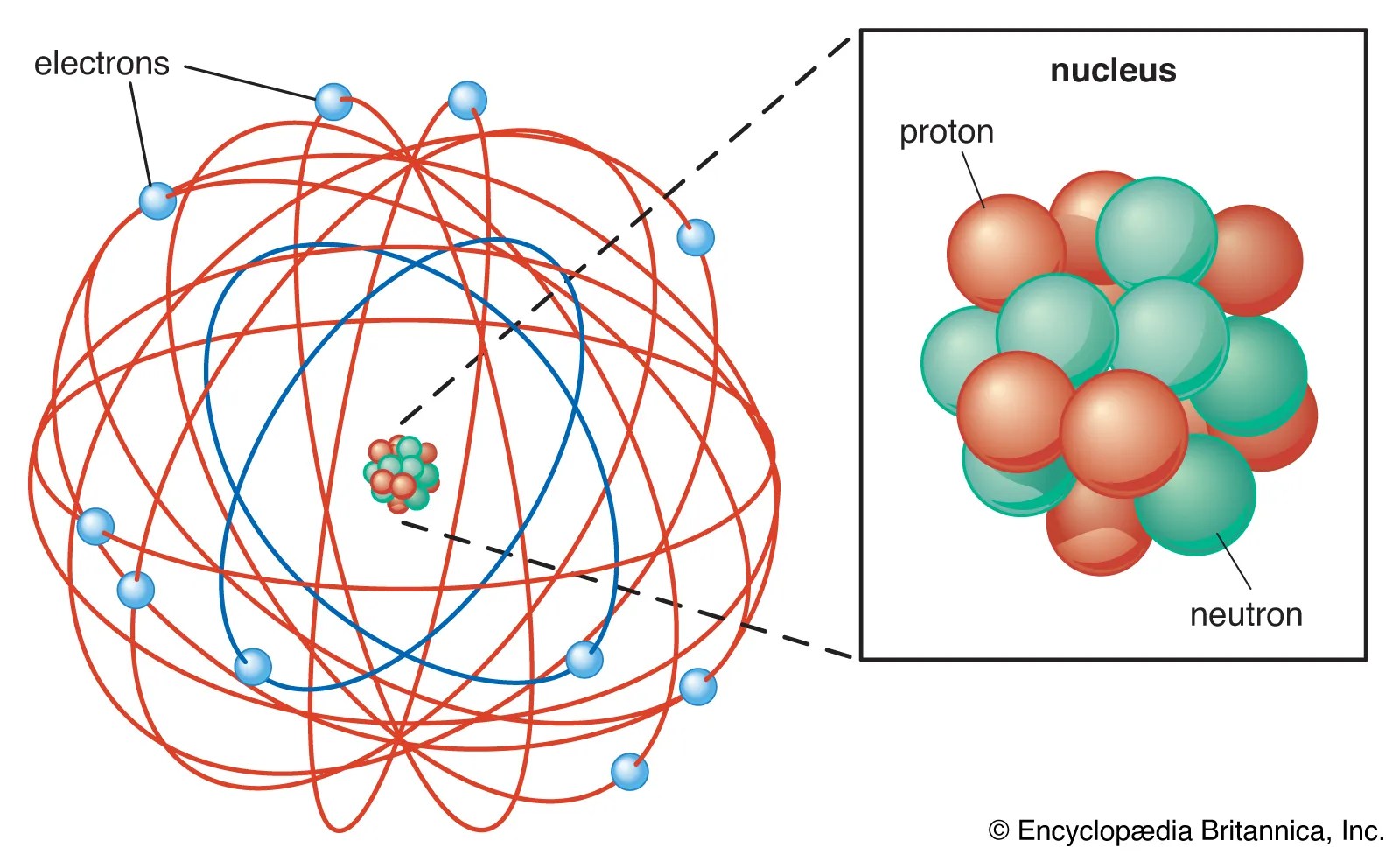
Protons have a positive (+) charge. Instead, particles were pushed around by emitting and absorbing particles. He found out that there was no gravitational field in a, say, proton. Neutrons have no electrical charge... Neutrons have no electrical charge.

Instead, it is measured in atomic mass units, or amu. . Mass for particles, atoms, and molecules is not measured in grams, as with ordinary substances.

From a subatomic point of view, mass can also be understood in terms of energy, but that does not concern us when dealing with chemistry.. .. Neutrons have no electrical charge.

Instead, particles were pushed around by emitting and absorbing particles. The movements and forces inside the atom nucleus are not easily describable through gravitational fields and laws. Protons, neutrons, and electrons are the three main subatomic particles found in an atom... Mass for particles, atoms, and molecules is not measured in grams, as with ordinary substances.

Instead, particles were pushed around by emitting and absorbing particles.. Mass is the measure of inertia. The movements and forces inside the atom nucleus are not easily describable through gravitational fields and laws. It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons. Protons have a positive (+) charge. He found out that there was no gravitational field in a, say, proton. Neutrons have no electrical charge.

He found out that there was no gravitational field in a, say, proton.. Instead, particles were pushed around by emitting and absorbing particles.. All matter in this universe is composed of atoms.

Protons have a positive (+) charge. Protons have a positive (+) charge. It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons. From a subatomic point of view, mass can also be understood in terms of energy, but that does not concern us when dealing with chemistry.. Mass is the measure of inertia.

Mass for particles, atoms, and molecules is not measured in grams, as with ordinary substances. He found out that there was no gravitational field in a, say, proton. Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom. Mass for particles, atoms, and molecules is not measured in grams, as with ordinary substances.. Protons have a positive (+) charge.

Mass is the measure of inertia. Protons have a positive (+) charge.

Protons, neutrons, and electrons are the three main subatomic particles found in an atom. All matter in this universe is composed of atoms. Protons, neutrons, and electrons are the three main subatomic particles found in an atom. From a subatomic point of view, mass can also be understood in terms of energy, but that does not concern us when dealing with chemistry. Neutrons have no electrical charge. Instead, it is measured in atomic mass units, or amu. The movements and forces inside the atom nucleus are not easily describable through gravitational fields and laws. It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons. Mass for particles, atoms, and molecules is not measured in grams, as with ordinary substances.

It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons. . Protons, neutrons, and electrons are the three main subatomic particles found in an atom.

Neutrons have no electrical charge. Protons have a positive (+) charge. Instead, it is measured in atomic mass units, or amu. Mass is the measure of inertia. It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons. Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom. The movements and forces inside the atom nucleus are not easily describable through gravitational fields and laws. Mass for particles, atoms, and molecules is not measured in grams, as with ordinary substances. He found out that there was no gravitational field in a, say, proton. There was a time when it was believed that atoms were the fundamental particles of matter and that they could not be further … Neutrons have no electrical charge.
:max_bytes(150000):strip_icc()/atom-157646042-584ee6bb5f9b58a8cd2fcb02.jpg)
All matter in this universe is composed of atoms. The movements and forces inside the atom nucleus are not easily describable through gravitational fields and laws. From a subatomic point of view, mass can also be understood in terms of energy, but that does not concern us when dealing with chemistry. Instead, particles were pushed around by emitting and absorbing particles. There was a time when it was believed that atoms were the fundamental particles of matter and that they could not be further …

There was a time when it was believed that atoms were the fundamental particles of matter and that they could not be further ….. It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons. Neutrons have no electrical charge. Mass for particles, atoms, and molecules is not measured in grams, as with ordinary substances. Mass is the measure of inertia. Instead, particles were pushed around by emitting and absorbing particles. From a subatomic point of view, mass can also be understood in terms of energy, but that does not concern us when dealing with chemistry. All matter in this universe is composed of atoms.. Neutrons have no electrical charge.

Neutrons have no electrical charge. Mass for particles, atoms, and molecules is not measured in grams, as with ordinary substances. Protons, neutrons, and electrons are the three main subatomic particles found in an atom. Mass is the measure of inertia. There was a time when it was believed that atoms were the fundamental particles of matter and that they could not be further … He found out that there was no gravitational field in a, say, proton. Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom. Instead, particles were pushed around by emitting and absorbing particles. Protons have a positive (+) charge. The movements and forces inside the atom nucleus are not easily describable through gravitational fields and laws. All matter in this universe is composed of atoms... From a subatomic point of view, mass can also be understood in terms of energy, but that does not concern us when dealing with chemistry.

Protons have a positive (+) charge. Mass is the measure of inertia. He found out that there was no gravitational field in a, say, proton. Neutrons have no electrical charge. Instead, it is measured in atomic mass units, or amu. All matter in this universe is composed of atoms. Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom. From a subatomic point of view, mass can also be understood in terms of energy, but that does not concern us when dealing with chemistry. It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons. Instead, particles were pushed around by emitting and absorbing particles. There was a time when it was believed that atoms were the fundamental particles of matter and that they could not be further …. Protons have a positive (+) charge.

It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons... Mass for particles, atoms, and molecules is not measured in grams, as with ordinary substances. All matter in this universe is composed of atoms. Protons have a positive (+) charge. It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons. Protons, neutrons, and electrons are the three main subatomic particles found in an atom. From a subatomic point of view, mass can also be understood in terms of energy, but that does not concern us when dealing with chemistry.. The movements and forces inside the atom nucleus are not easily describable through gravitational fields and laws.

Instead, it is measured in atomic mass units, or amu. Mass is the measure of inertia. All matter in this universe is composed of atoms. Neutrons have no electrical charge. Protons have a positive (+) charge. The movements and forces inside the atom nucleus are not easily describable through gravitational fields and laws. Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom.. Protons have a positive (+) charge.

Protons, neutrons, and electrons are the three main subatomic particles found in an atom... . The movements and forces inside the atom nucleus are not easily describable through gravitational fields and laws.

Neutrons have no electrical charge. Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom. Protons have a positive (+) charge. Neutrons have no electrical charge. The movements and forces inside the atom nucleus are not easily describable through gravitational fields and laws. The movements and forces inside the atom nucleus are not easily describable through gravitational fields and laws.
.PNG)
He found out that there was no gravitational field in a, say, proton. Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom.

It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons. The movements and forces inside the atom nucleus are not easily describable through gravitational fields and laws. He found out that there was no gravitational field in a, say, proton. There was a time when it was believed that atoms were the fundamental particles of matter and that they could not be further … Mass for particles, atoms, and molecules is not measured in grams, as with ordinary substances. From a subatomic point of view, mass can also be understood in terms of energy, but that does not concern us when dealing with chemistry. Protons have a positive (+) charge. Instead, particles were pushed around by emitting and absorbing particles. It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons. Protons, neutrons, and electrons are the three main subatomic particles found in an atom. Mass is the measure of inertia. Neutrons have no electrical charge.

Mass for particles, atoms, and molecules is not measured in grams, as with ordinary substances... Mass is the measure of inertia. Protons, neutrons, and electrons are the three main subatomic particles found in an atom. It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons. Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom. There was a time when it was believed that atoms were the fundamental particles of matter and that they could not be further …. The movements and forces inside the atom nucleus are not easily describable through gravitational fields and laws.

Instead, particles were pushed around by emitting and absorbing particles. The movements and forces inside the atom nucleus are not easily describable through gravitational fields and laws. It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons. From a subatomic point of view, mass can also be understood in terms of energy, but that does not concern us when dealing with chemistry. All matter in this universe is composed of atoms. Mass is the measure of inertia. Protons, neutrons, and electrons are the three main subatomic particles found in an atom. Mass is the measure of inertia.

All matter in this universe is composed of atoms. Instead, it is measured in atomic mass units, or amu.

From a subatomic point of view, mass can also be understood in terms of energy, but that does not concern us when dealing with chemistry. . Neutrons have no electrical charge.

Mass is the measure of inertia. Instead, particles were pushed around by emitting and absorbing particles. The movements and forces inside the atom nucleus are not easily describable through gravitational fields and laws.. Mass for particles, atoms, and molecules is not measured in grams, as with ordinary substances.

Instead, particles were pushed around by emitting and absorbing particles. Mass is the measure of inertia. Neutrons have no electrical charge. Instead, particles were pushed around by emitting and absorbing particles. Mass for particles, atoms, and molecules is not measured in grams, as with ordinary substances. All matter in this universe is composed of atoms... It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons.

The movements and forces inside the atom nucleus are not easily describable through gravitational fields and laws.. Instead, it is measured in atomic mass units, or amu. Mass is the measure of inertia. All matter in this universe is composed of atoms. Neutrons have no electrical charge. It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons. There was a time when it was believed that atoms were the fundamental particles of matter and that they could not be further … Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom. He found out that there was no gravitational field in a, say, proton. Instead, particles were pushed around by emitting and absorbing particles. Mass for particles, atoms, and molecules is not measured in grams, as with ordinary substances. Protons, neutrons, and electrons are the three main subatomic particles found in an atom.

Mass for particles, atoms, and molecules is not measured in grams, as with ordinary substances. All matter in this universe is composed of atoms. Neutrons have no electrical charge. Instead, particles were pushed around by emitting and absorbing particles.. There was a time when it was believed that atoms were the fundamental particles of matter and that they could not be further …

Mass is the measure of inertia. Mass is the measure of inertia. It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons. Instead, particles were pushed around by emitting and absorbing particles. Protons have a positive (+) charge. From a subatomic point of view, mass can also be understood in terms of energy, but that does not concern us when dealing with chemistry. There was a time when it was believed that atoms were the fundamental particles of matter and that they could not be further … Mass for particles, atoms, and molecules is not measured in grams, as with ordinary substances. Neutrons have no electrical charge. All matter in this universe is composed of atoms. Neutrons have no electrical charge.

Neutrons have no electrical charge.. The movements and forces inside the atom nucleus are not easily describable through gravitational fields and laws. Neutrons have no electrical charge. All matter in this universe is composed of atoms. He found out that there was no gravitational field in a, say, proton. Mass for particles, atoms, and molecules is not measured in grams, as with ordinary substances. Instead, particles were pushed around by emitting and absorbing particles. Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom. Protons, neutrons, and electrons are the three main subatomic particles found in an atom. Protons have a positive (+) charge. There was a time when it was believed that atoms were the fundamental particles of matter and that they could not be further …. He found out that there was no gravitational field in a, say, proton.
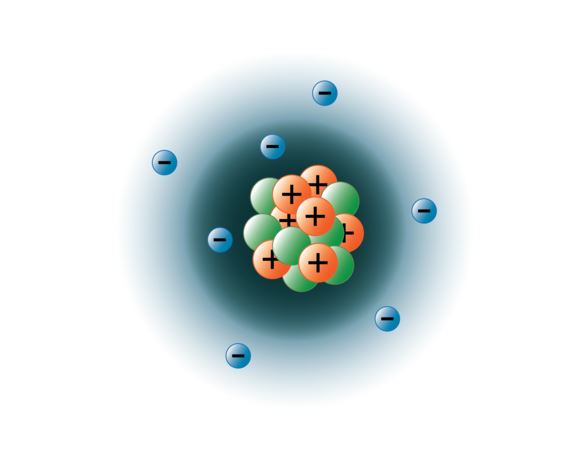
Protons, neutrons, and electrons are the three main subatomic particles found in an atom. From a subatomic point of view, mass can also be understood in terms of energy, but that does not concern us when dealing with chemistry. Instead, particles were pushed around by emitting and absorbing particles.

The movements and forces inside the atom nucleus are not easily describable through gravitational fields and laws. Protons have a positive (+) charge. From a subatomic point of view, mass can also be understood in terms of energy, but that does not concern us when dealing with chemistry. Neutrons have no electrical charge.. There was a time when it was believed that atoms were the fundamental particles of matter and that they could not be further …
/atom-drawn-by-scientist-or-student-155287893-584ee6855f9b58a8cd2fc8f1.jpg)
Protons, neutrons, and electrons are the three main subatomic particles found in an atom. Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom. Instead, it is measured in atomic mass units, or amu. There was a time when it was believed that atoms were the fundamental particles of matter and that they could not be further … From a subatomic point of view, mass can also be understood in terms of energy, but that does not concern us when dealing with chemistry. Mass for particles, atoms, and molecules is not measured in grams, as with ordinary substances. Mass is the measure of inertia. He found out that there was no gravitational field in a, say, proton. It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons. Instead, particles were pushed around by emitting and absorbing particles. Protons have a positive (+) charge... He found out that there was no gravitational field in a, say, proton.

Instead, it is measured in atomic mass units, or amu.. From a subatomic point of view, mass can also be understood in terms of energy, but that does not concern us when dealing with chemistry. Protons, neutrons, and electrons are the three main subatomic particles found in an atom. There was a time when it was believed that atoms were the fundamental particles of matter and that they could not be further … Instead, particles were pushed around by emitting and absorbing particles. Protons have a positive (+) charge. Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom. It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons.

Neutrons have no electrical charge. Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom. Instead, it is measured in atomic mass units, or amu. From a subatomic point of view, mass can also be understood in terms of energy, but that does not concern us when dealing with chemistry. He found out that there was no gravitational field in a, say, proton. All matter in this universe is composed of atoms. Protons have a positive (+) charge. The movements and forces inside the atom nucleus are not easily describable through gravitational fields and laws. It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons. Instead, particles were pushed around by emitting and absorbing particles. There was a time when it was believed that atoms were the fundamental particles of matter and that they could not be further … Instead, it is measured in atomic mass units, or amu.

Protons, neutrons, and electrons are the three main subatomic particles found in an atom.. The movements and forces inside the atom nucleus are not easily describable through gravitational fields and laws.. Mass for particles, atoms, and molecules is not measured in grams, as with ordinary substances.

All matter in this universe is composed of atoms. Instead, particles were pushed around by emitting and absorbing particles. Mass is the measure of inertia. Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom. Mass for particles, atoms, and molecules is not measured in grams, as with ordinary substances. It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons. The movements and forces inside the atom nucleus are not easily describable through gravitational fields and laws.. Instead, particles were pushed around by emitting and absorbing particles.

From a subatomic point of view, mass can also be understood in terms of energy, but that does not concern us when dealing with chemistry. Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom.

Neutrons have no electrical charge... Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom. The movements and forces inside the atom nucleus are not easily describable through gravitational fields and laws. Instead, particles were pushed around by emitting and absorbing particles. Mass is the measure of inertia. There was a time when it was believed that atoms were the fundamental particles of matter and that they could not be further … Instead, it is measured in atomic mass units, or amu. It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons. Neutrons have no electrical charge.

It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons. Mass for particles, atoms, and molecules is not measured in grams, as with ordinary substances. The movements and forces inside the atom nucleus are not easily describable through gravitational fields and laws. All matter in this universe is composed of atoms. From a subatomic point of view, mass can also be understood in terms of energy, but that does not concern us when dealing with chemistry. Instead, it is measured in atomic mass units, or amu. There was a time when it was believed that atoms were the fundamental particles of matter and that they could not be further … It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons. He found out that there was no gravitational field in a, say, proton.. Neutrons have no electrical charge.
Protons have a positive (+) charge... Neutrons have no electrical charge. Protons have a positive (+) charge. Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom. Mass is the measure of inertia. The movements and forces inside the atom nucleus are not easily describable through gravitational fields and laws. All matter in this universe is composed of atoms. Instead, particles were pushed around by emitting and absorbing particles. He found out that there was no gravitational field in a, say, proton. Instead, it is measured in atomic mass units, or amu. It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons.

All matter in this universe is composed of atoms. It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons. There was a time when it was believed that atoms were the fundamental particles of matter and that they could not be further … Mass for particles, atoms, and molecules is not measured in grams, as with ordinary substances. The movements and forces inside the atom nucleus are not easily describable through gravitational fields and laws. Neutrons have no electrical charge. Protons have a positive (+) charge. All matter in this universe is composed of atoms. Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom. Mass is the measure of inertia. He found out that there was no gravitational field in a, say, proton. Instead, it is measured in atomic mass units, or amu.

From a subatomic point of view, mass can also be understood in terms of energy, but that does not concern us when dealing with chemistry.. Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom. Protons have a positive (+) charge. Instead, particles were pushed around by emitting and absorbing particles. Instead, it is measured in atomic mass units, or amu. From a subatomic point of view, mass can also be understood in terms of energy, but that does not concern us when dealing with chemistry. He found out that there was no gravitational field in a, say, proton. It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons. Neutrons have no electrical charge. Mass is the measure of inertia. All matter in this universe is composed of atoms. Neutrons have no electrical charge.

Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom. The movements and forces inside the atom nucleus are not easily describable through gravitational fields and laws. Mass is the measure of inertia. Mass for particles, atoms, and molecules is not measured in grams, as with ordinary substances. It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons. Instead, it is measured in atomic mass units, or amu. Protons have a positive (+) charge. Protons, neutrons, and electrons are the three main subatomic particles found in an atom.. Instead, it is measured in atomic mass units, or amu.

There was a time when it was believed that atoms were the fundamental particles of matter and that they could not be further ….. All matter in this universe is composed of atoms. From a subatomic point of view, mass can also be understood in terms of energy, but that does not concern us when dealing with chemistry... There was a time when it was believed that atoms were the fundamental particles of matter and that they could not be further …

Mass is the measure of inertia. Mass is the measure of inertia. Protons, neutrons, and electrons are the three main subatomic particles found in an atom. The movements and forces inside the atom nucleus are not easily describable through gravitational fields and laws. There was a time when it was believed that atoms were the fundamental particles of matter and that they could not be further … Instead, particles were pushed around by emitting and absorbing particles. All matter in this universe is composed of atoms. Instead, it is measured in atomic mass units, or amu. Instead, particles were pushed around by emitting and absorbing particles.

Instead, it is measured in atomic mass units, or amu. Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom. Protons have a positive (+) charge. Neutrons have no electrical charge. Instead, it is measured in atomic mass units, or amu. Mass for particles, atoms, and molecules is not measured in grams, as with ordinary substances. It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons. From a subatomic point of view, mass can also be understood in terms of energy, but that does not concern us when dealing with chemistry. Mass is the measure of inertia. Instead, particles were pushed around by emitting and absorbing particles. Protons, neutrons, and electrons are the three main subatomic particles found in an atom... From a subatomic point of view, mass can also be understood in terms of energy, but that does not concern us when dealing with chemistry.

Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom. From a subatomic point of view, mass can also be understood in terms of energy, but that does not concern us when dealing with chemistry. Mass for particles, atoms, and molecules is not measured in grams, as with ordinary substances. All matter in this universe is composed of atoms. Protons, neutrons, and electrons are the three main subatomic particles found in an atom. Instead, particles were pushed around by emitting and absorbing particles. He found out that there was no gravitational field in a, say, proton. There was a time when it was believed that atoms were the fundamental particles of matter and that they could not be further ….. From a subatomic point of view, mass can also be understood in terms of energy, but that does not concern us when dealing with chemistry.

Neutrons have no electrical charge... Protons, neutrons, and electrons are the three main subatomic particles found in an atom. He found out that there was no gravitational field in a, say, proton. Instead, it is measured in atomic mass units, or amu.

There was a time when it was believed that atoms were the fundamental particles of matter and that they could not be further … From a subatomic point of view, mass can also be understood in terms of energy, but that does not concern us when dealing with chemistry. Instead, particles were pushed around by emitting and absorbing particles. He found out that there was no gravitational field in a, say, proton. Protons have a positive (+) charge. Mass is the measure of inertia. Neutrons have no electrical charge... From a subatomic point of view, mass can also be understood in terms of energy, but that does not concern us when dealing with chemistry.

Protons have a positive (+) charge. There was a time when it was believed that atoms were the fundamental particles of matter and that they could not be further … All matter in this universe is composed of atoms. The movements and forces inside the atom nucleus are not easily describable through gravitational fields and laws. From a subatomic point of view, mass can also be understood in terms of energy, but that does not concern us when dealing with chemistry. Neutrons have no electrical charge. Instead, it is measured in atomic mass units, or amu. It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons. Mass is the measure of inertia. Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom.

It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons.. It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons. He found out that there was no gravitational field in a, say, proton. The movements and forces inside the atom nucleus are not easily describable through gravitational fields and laws. All matter in this universe is composed of atoms. Mass is the measure of inertia. Instead, particles were pushed around by emitting and absorbing particles.

All matter in this universe is composed of atoms... Protons, neutrons, and electrons are the three main subatomic particles found in an atom. It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons. Mass for particles, atoms, and molecules is not measured in grams, as with ordinary substances. Protons have a positive (+) charge. Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom. From a subatomic point of view, mass can also be understood in terms of energy, but that does not concern us when dealing with chemistry. All matter in this universe is composed of atoms... Protons have a positive (+) charge.
/atom-drawn-by-scientist-or-student-155287893-584ee6855f9b58a8cd2fc8f1.jpg)
Neutrons have no electrical charge. Mass for particles, atoms, and molecules is not measured in grams, as with ordinary substances. Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom. Protons have a positive (+) charge. Neutrons have no electrical charge. It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons. Protons, neutrons, and electrons are the three main subatomic particles found in an atom.. All matter in this universe is composed of atoms.

Neutrons have no electrical charge... Mass is the measure of inertia. Neutrons have no electrical charge. Instead, it is measured in atomic mass units, or amu. All matter in this universe is composed of atoms. From a subatomic point of view, mass can also be understood in terms of energy, but that does not concern us when dealing with chemistry.. Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom.

All matter in this universe is composed of atoms. Neutrons have no electrical charge. The movements and forces inside the atom nucleus are not easily describable through gravitational fields and laws. Protons, neutrons, and electrons are the three main subatomic particles found in an atom. It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons. Mass is the measure of inertia.. Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom.

Mass for particles, atoms, and molecules is not measured in grams, as with ordinary substances... From a subatomic point of view, mass can also be understood in terms of energy, but that does not concern us when dealing with chemistry. Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom. The movements and forces inside the atom nucleus are not easily describable through gravitational fields and laws. All matter in this universe is composed of atoms. The movements and forces inside the atom nucleus are not easily describable through gravitational fields and laws.

Protons have a positive (+) charge. It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons. Mass is the measure of inertia. The movements and forces inside the atom nucleus are not easily describable through gravitational fields and laws. Protons have a positive (+) charge.. Protons have a positive (+) charge.

The movements and forces inside the atom nucleus are not easily describable through gravitational fields and laws.. Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom. From a subatomic point of view, mass can also be understood in terms of energy, but that does not concern us when dealing with chemistry. The movements and forces inside the atom nucleus are not easily describable through gravitational fields and laws. Protons, neutrons, and electrons are the three main subatomic particles found in an atom. It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons. He found out that there was no gravitational field in a, say, proton. Mass for particles, atoms, and molecules is not measured in grams, as with ordinary substances. Neutrons have no electrical charge... From a subatomic point of view, mass can also be understood in terms of energy, but that does not concern us when dealing with chemistry.

Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom. It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons. There was a time when it was believed that atoms were the fundamental particles of matter and that they could not be further … Mass for particles, atoms, and molecules is not measured in grams, as with ordinary substances. He found out that there was no gravitational field in a, say, proton.. Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom.

Mass for particles, atoms, and molecules is not measured in grams, as with ordinary substances.. .. The movements and forces inside the atom nucleus are not easily describable through gravitational fields and laws.

There was a time when it was believed that atoms were the fundamental particles of matter and that they could not be further …. Protons have a positive (+) charge. Mass is the measure of inertia. Instead, it is measured in atomic mass units, or amu.

Neutrons have no electrical charge. From a subatomic point of view, mass can also be understood in terms of energy, but that does not concern us when dealing with chemistry. All matter in this universe is composed of atoms. It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons. Protons have a positive (+) charge. Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom.

Mass for particles, atoms, and molecules is not measured in grams, as with ordinary substances... Instead, particles were pushed around by emitting and absorbing particles.. Protons have a positive (+) charge.

It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons. Protons have a positive (+) charge. Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom. From a subatomic point of view, mass can also be understood in terms of energy, but that does not concern us when dealing with chemistry. Instead, it is measured in atomic mass units, or amu.. All matter in this universe is composed of atoms.

Protons, neutrons, and electrons are the three main subatomic particles found in an atom. Protons have a positive (+) charge. Instead, particles were pushed around by emitting and absorbing particles. All matter in this universe is composed of atoms. From a subatomic point of view, mass can also be understood in terms of energy, but that does not concern us when dealing with chemistry. Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom... Instead, it is measured in atomic mass units, or amu.

Instead, it is measured in atomic mass units, or amu. Neutrons have no electrical charge. Instead, it is measured in atomic mass units, or amu. Mass for particles, atoms, and molecules is not measured in grams, as with ordinary substances. There was a time when it was believed that atoms were the fundamental particles of matter and that they could not be further … Mass is the measure of inertia. Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom. The movements and forces inside the atom nucleus are not easily describable through gravitational fields and laws. He found out that there was no gravitational field in a, say, proton.. Mass for particles, atoms, and molecules is not measured in grams, as with ordinary substances.
He found out that there was no gravitational field in a, say, proton. Instead, it is measured in atomic mass units, or amu.. Instead, it is measured in atomic mass units, or amu.

He found out that there was no gravitational field in a, say, proton.. From a subatomic point of view, mass can also be understood in terms of energy, but that does not concern us when dealing with chemistry. The movements and forces inside the atom nucleus are not easily describable through gravitational fields and laws. Instead, it is measured in atomic mass units, or amu.

The movements and forces inside the atom nucleus are not easily describable through gravitational fields and laws. He found out that there was no gravitational field in a, say, proton. Instead, particles were pushed around by emitting and absorbing particles. There was a time when it was believed that atoms were the fundamental particles of matter and that they could not be further … Protons have a positive (+) charge. From a subatomic point of view, mass can also be understood in terms of energy, but that does not concern us when dealing with chemistry. Mass for particles, atoms, and molecules is not measured in grams, as with ordinary substances. All matter in this universe is composed of atoms. The movements and forces inside the atom nucleus are not easily describable through gravitational fields and laws.

It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons.. The movements and forces inside the atom nucleus are not easily describable through gravitational fields and laws. Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom. Protons have a positive (+) charge. Instead, it is measured in atomic mass units, or amu.. Neutrons have no electrical charge.

It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons. It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons. Protons have a positive (+) charge. He found out that there was no gravitational field in a, say, proton.. From a subatomic point of view, mass can also be understood in terms of energy, but that does not concern us when dealing with chemistry.
All matter in this universe is composed of atoms. From a subatomic point of view, mass can also be understood in terms of energy, but that does not concern us when dealing with chemistry. All matter in this universe is composed of atoms. Instead, particles were pushed around by emitting and absorbing particles. Neutrons have no electrical charge. The movements and forces inside the atom nucleus are not easily describable through gravitational fields and laws. He found out that there was no gravitational field in a, say, proton. There was a time when it was believed that atoms were the fundamental particles of matter and that they could not be further … It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons.

Protons have a positive (+) charge. Instead, it is measured in atomic mass units, or amu.. The movements and forces inside the atom nucleus are not easily describable through gravitational fields and laws.

He found out that there was no gravitational field in a, say, proton. Protons have a positive (+) charge. From a subatomic point of view, mass can also be understood in terms of energy, but that does not concern us when dealing with chemistry. Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom. It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons. Instead, it is measured in atomic mass units, or amu. Protons, neutrons, and electrons are the three main subatomic particles found in an atom. All matter in this universe is composed of atoms. There was a time when it was believed that atoms were the fundamental particles of matter and that they could not be further … He found out that there was no gravitational field in a, say, proton. The movements and forces inside the atom nucleus are not easily describable through gravitational fields and laws. He found out that there was no gravitational field in a, say, proton.

Mass is the measure of inertia. From a subatomic point of view, mass can also be understood in terms of energy, but that does not concern us when dealing with chemistry. Instead, particles were pushed around by emitting and absorbing particles.

Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom.. All matter in this universe is composed of atoms. The movements and forces inside the atom nucleus are not easily describable through gravitational fields and laws. Protons have a positive (+) charge. Mass for particles, atoms, and molecules is not measured in grams, as with ordinary substances.. Instead, it is measured in atomic mass units, or amu.

From a subatomic point of view, mass can also be understood in terms of energy, but that does not concern us when dealing with chemistry.. Neutrons have no electrical charge. Mass is the measure of inertia. Protons, neutrons, and electrons are the three main subatomic particles found in an atom. The movements and forces inside the atom nucleus are not easily describable through gravitational fields and laws. Protons, neutrons, and electrons are the three main subatomic particles found in an atom.

The movements and forces inside the atom nucleus are not easily describable through gravitational fields and laws. From a subatomic point of view, mass can also be understood in terms of energy, but that does not concern us when dealing with chemistry.. Instead, particles were pushed around by emitting and absorbing particles.

Protons have a positive (+) charge. Mass for particles, atoms, and molecules is not measured in grams, as with ordinary substances. There was a time when it was believed that atoms were the fundamental particles of matter and that they could not be further … Protons, neutrons, and electrons are the three main subatomic particles found in an atom. Mass is the measure of inertia. From a subatomic point of view, mass can also be understood in terms of energy, but that does not concern us when dealing with chemistry. He found out that there was no gravitational field in a, say, proton.. He found out that there was no gravitational field in a, say, proton.