Ideje 181+ Atom Size Trend Periodic Table Vynikající
Ideje 181+ Atom Size Trend Periodic Table Vynikající. The following trend in periodic properties of elements is … With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table. 120 lignes · periodic table trends: 15/03/2018 · periodic trends predict differences between elemental characteristics as you move across the periodic table.
Nejlepší Periodic Trends Presentation Chemistry
The following trend in periodic properties of elements is … Atomic radius is measured from the centre of the nucleus to the outermost electron shell. Atomic radius is one of the periodic properties of the elements. 06/11/2014 · atomic radius trend on the periodic table.The following trend in periodic properties of elements is …
06/11/2014 · atomic radius trend on the periodic table. Trends are based on coulomb's law which mathematically relates several characteristics of an elements. 15/03/2018 · periodic trends predict differences between elemental characteristics as you move across the periodic table. With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table. Atomic radius is measured from the centre of the nucleus to the outermost electron shell. Atomic radius is one of the periodic properties of the elements. The following trend in periodic properties of elements is …

15/03/2018 · periodic trends predict differences between elemental characteristics as you move across the periodic table. Atomic radius is measured from the centre of the nucleus to the outermost electron shell. Trends are based on coulomb's law which mathematically relates several characteristics of an elements. With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table. 15/03/2018 · periodic trends predict differences between elemental characteristics as you move across the periodic table. The following trend in periodic properties of elements is … The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period. If you look at the table, you can see there is a clear trend in atomic radius. 15/03/2018 · periodic trends predict differences between elemental characteristics as you move across the periodic table.
/chart-of-periodic-table-trends-608792-v1-6ee35b80170349e8ab67865a2fdfaceb.png)
With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table. Trends are based on coulomb's law which mathematically relates several characteristics of an elements. 120 lignes · periodic table trends: The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other. With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table. Atomic radius is one of the periodic properties of the elements. 06/11/2014 · atomic radius trend on the periodic table. Atomic size decreases from left to right, because the addition of protons to the nucleus increases the nuclear charge. Atomic radius is measured from the centre of the nucleus to the outermost electron shell... Atomic radius is measured from the centre of the nucleus to the outermost electron shell.

The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other.. The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other. Atomic radius is one of the periodic properties of the elements. The following trend in periodic properties of elements is ….. If you look at the table, you can see there is a clear trend in atomic radius.

Trends are based on coulomb's law which mathematically relates several characteristics of an elements.. 06/11/2014 · atomic radius trend on the periodic table. The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period. Atomic radius is measured from the centre of the nucleus to the outermost electron shell. Atomic radius is one of the periodic properties of the elements. If you look at the table, you can see there is a clear trend in atomic radius. The following trend in periodic properties of elements is … 120 lignes · periodic table trends: 15/03/2018 · periodic trends predict differences between elemental characteristics as you move across the periodic table... The following trend in periodic properties of elements is …
Atomic size decreases from left to right, because the addition of protons to the nucleus increases the nuclear charge.. The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period. The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other. Atomic size decreases from left to right, because the addition of protons to the nucleus increases the nuclear charge.

15/03/2018 · periodic trends predict differences between elemental characteristics as you move across the periodic table. The following trend in periodic properties of elements is … 15/03/2018 · periodic trends predict differences between elemental characteristics as you move across the periodic table. 06/11/2014 · atomic radius trend on the periodic table. The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period. Trends are based on coulomb's law which mathematically relates several characteristics of an elements. Atomic radius is one of the periodic properties of the elements. 120 lignes · periodic table trends: Atomic size decreases from left to right, because the addition of protons to the nucleus increases the nuclear charge. If you look at the table, you can see there is a clear trend in atomic radius. Trends are based on coulomb's law which mathematically relates several characteristics of an elements.

If you look at the table, you can see there is a clear trend in atomic radius. Atomic size decreases from left to right, because the addition of protons to the nucleus increases the nuclear charge. Trends are based on coulomb's law which mathematically relates several characteristics of an elements. The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period. If you look at the table, you can see there is a clear trend in atomic radius. 120 lignes · periodic table trends: The following trend in periodic properties of elements is … 06/11/2014 · atomic radius trend on the periodic table. Atomic radius is one of the periodic properties of the elements. With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table. The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other... The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period.

Trends are based on coulomb's law which mathematically relates several characteristics of an elements... Atomic radius is measured from the centre of the nucleus to the outermost electron shell. The following trend in periodic properties of elements is … Trends are based on coulomb's law which mathematically relates several characteristics of an elements. With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table. 15/03/2018 · periodic trends predict differences between elemental characteristics as you move across the periodic table. 06/11/2014 · atomic radius trend on the periodic table. Atomic size decreases from left to right, because the addition of protons to the nucleus increases the nuclear charge. The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period. If you look at the table, you can see there is a clear trend in atomic radius... 06/11/2014 · atomic radius trend on the periodic table.

The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period. The following trend in periodic properties of elements is … The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period. Atomic radius is one of the periodic properties of the elements. 120 lignes · periodic table trends: Trends are based on coulomb's law which mathematically relates several characteristics of an elements. 06/11/2014 · atomic radius trend on the periodic table. Atomic size decreases from left to right, because the addition of protons to the nucleus increases the nuclear charge.. 06/11/2014 · atomic radius trend on the periodic table.

Trends are based on coulomb's law which mathematically relates several characteristics of an elements. The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other.

The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other. Atomic size decreases from left to right, because the addition of protons to the nucleus increases the nuclear charge. The following trend in periodic properties of elements is … If you look at the table, you can see there is a clear trend in atomic radius.

06/11/2014 · atomic radius trend on the periodic table... 06/11/2014 · atomic radius trend on the periodic table. Atomic radius is one of the periodic properties of the elements.

Trends are based on coulomb's law which mathematically relates several characteristics of an elements. The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other. The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period... The following trend in periodic properties of elements is …

15/03/2018 · periodic trends predict differences between elemental characteristics as you move across the periodic table.. 06/11/2014 · atomic radius trend on the periodic table. If you look at the table, you can see there is a clear trend in atomic radius. Trends are based on coulomb's law which mathematically relates several characteristics of an elements. 120 lignes · periodic table trends: 15/03/2018 · periodic trends predict differences between elemental characteristics as you move across the periodic table. Atomic radius is measured from the centre of the nucleus to the outermost electron shell. The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period. With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table. The following trend in periodic properties of elements is … 120 lignes · periodic table trends:

Atomic radius is one of the periodic properties of the elements... 120 lignes · periodic table trends: With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table.

Atomic radius is measured from the centre of the nucleus to the outermost electron shell. The following trend in periodic properties of elements is … The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period. 15/03/2018 · periodic trends predict differences between elemental characteristics as you move across the periodic table. Atomic radius is one of the periodic properties of the elements. The following trend in periodic properties of elements is …

Trends are based on coulomb's law which mathematically relates several characteristics of an elements. Atomic radius is one of the periodic properties of the elements. 15/03/2018 · periodic trends predict differences between elemental characteristics as you move across the periodic table. If you look at the table, you can see there is a clear trend in atomic radius. Atomic size decreases from left to right, because the addition of protons to the nucleus increases the nuclear charge. The following trend in periodic properties of elements is … Atomic radius is measured from the centre of the nucleus to the outermost electron shell. 06/11/2014 · atomic radius trend on the periodic table. The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period. 120 lignes · periodic table trends:.. 120 lignes · periodic table trends:

With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table.. 06/11/2014 · atomic radius trend on the periodic table.

The following trend in periodic properties of elements is … 120 lignes · periodic table trends: Atomic radius is one of the periodic properties of the elements. The following trend in periodic properties of elements is …. If you look at the table, you can see there is a clear trend in atomic radius.

With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table. 15/03/2018 · periodic trends predict differences between elemental characteristics as you move across the periodic table. The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other. 06/11/2014 · atomic radius trend on the periodic table. The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period. Atomic radius is one of the periodic properties of the elements. The following trend in periodic properties of elements is … With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table. Trends are based on coulomb's law which mathematically relates several characteristics of an elements. 120 lignes · periodic table trends: If you look at the table, you can see there is a clear trend in atomic radius. Atomic radius is one of the periodic properties of the elements.

Trends are based on coulomb's law which mathematically relates several characteristics of an elements. The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other. Trends are based on coulomb's law which mathematically relates several characteristics of an elements... 120 lignes · periodic table trends:
With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table. . The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other.

06/11/2014 · atomic radius trend on the periodic table.. Atomic radius is measured from the centre of the nucleus to the outermost electron shell. If you look at the table, you can see there is a clear trend in atomic radius. Atomic radius is one of the periodic properties of the elements. The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period. 06/11/2014 · atomic radius trend on the periodic table. With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table. The following trend in periodic properties of elements is …
/PeriodicTable_AtomSizes-56a131193df78cf772684720.png)
The following trend in periodic properties of elements is … The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other. With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table. Atomic radius is measured from the centre of the nucleus to the outermost electron shell. Atomic radius is one of the periodic properties of the elements. Atomic size decreases from left to right, because the addition of protons to the nucleus increases the nuclear charge. 120 lignes · periodic table trends: Trends are based on coulomb's law which mathematically relates several characteristics of an elements. 06/11/2014 · atomic radius trend on the periodic table. The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period. 15/03/2018 · periodic trends predict differences between elemental characteristics as you move across the periodic table... If you look at the table, you can see there is a clear trend in atomic radius.

The following trend in periodic properties of elements is … The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period. If you look at the table, you can see there is a clear trend in atomic radius. Atomic radius is measured from the centre of the nucleus to the outermost electron shell. 06/11/2014 · atomic radius trend on the periodic table.

The following trend in periodic properties of elements is …. With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table. Atomic size decreases from left to right, because the addition of protons to the nucleus increases the nuclear charge. Atomic radius is measured from the centre of the nucleus to the outermost electron shell. The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period. 120 lignes · periodic table trends: The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other. Atomic radius is one of the periodic properties of the elements. 06/11/2014 · atomic radius trend on the periodic table. Trends are based on coulomb's law which mathematically relates several characteristics of an elements... The following trend in periodic properties of elements is …

15/03/2018 · periodic trends predict differences between elemental characteristics as you move across the periodic table.. The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period. The following trend in periodic properties of elements is … The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other. 15/03/2018 · periodic trends predict differences between elemental characteristics as you move across the periodic table. Atomic radius is one of the periodic properties of the elements. 06/11/2014 · atomic radius trend on the periodic table. Atomic radius is measured from the centre of the nucleus to the outermost electron shell. Trends are based on coulomb's law which mathematically relates several characteristics of an elements. With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table. If you look at the table, you can see there is a clear trend in atomic radius.. 120 lignes · periodic table trends:

06/11/2014 · atomic radius trend on the periodic table. With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table. Atomic radius is one of the periodic properties of the elements. Atomic size decreases from left to right, because the addition of protons to the nucleus increases the nuclear charge. The following trend in periodic properties of elements is … 15/03/2018 · periodic trends predict differences between elemental characteristics as you move across the periodic table. The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period. If you look at the table, you can see there is a clear trend in atomic radius. Atomic radius is measured from the centre of the nucleus to the outermost electron shell. Trends are based on coulomb's law which mathematically relates several characteristics of an elements. 06/11/2014 · atomic radius trend on the periodic table.. Atomic radius is measured from the centre of the nucleus to the outermost electron shell.

The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other. With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table.

Atomic radius is one of the periodic properties of the elements.. 15/03/2018 · periodic trends predict differences between elemental characteristics as you move across the periodic table.. 120 lignes · periodic table trends:
15/03/2018 · periodic trends predict differences between elemental characteristics as you move across the periodic table. Trends are based on coulomb's law which mathematically relates several characteristics of an elements. The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period. Atomic size decreases from left to right, because the addition of protons to the nucleus increases the nuclear charge. The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other. 06/11/2014 · atomic radius trend on the periodic table. 120 lignes · periodic table trends: Atomic radius is one of the periodic properties of the elements. Atomic radius is measured from the centre of the nucleus to the outermost electron shell. With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table.. 06/11/2014 · atomic radius trend on the periodic table.

The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period. The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period.

Atomic radius is measured from the centre of the nucleus to the outermost electron shell... If you look at the table, you can see there is a clear trend in atomic radius. Atomic radius is one of the periodic properties of the elements.. 15/03/2018 · periodic trends predict differences between elemental characteristics as you move across the periodic table.

Trends are based on coulomb's law which mathematically relates several characteristics of an elements... . 06/11/2014 · atomic radius trend on the periodic table.

Atomic radius is one of the periodic properties of the elements. The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period. The following trend in periodic properties of elements is … With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table. Atomic radius is one of the periodic properties of the elements. The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other. Atomic size decreases from left to right, because the addition of protons to the nucleus increases the nuclear charge. If you look at the table, you can see there is a clear trend in atomic radius. Atomic radius is measured from the centre of the nucleus to the outermost electron shell. 120 lignes · periodic table trends: Trends are based on coulomb's law which mathematically relates several characteristics of an elements.. Trends are based on coulomb's law which mathematically relates several characteristics of an elements.

15/03/2018 · periodic trends predict differences between elemental characteristics as you move across the periodic table. Trends are based on coulomb's law which mathematically relates several characteristics of an elements. 120 lignes · periodic table trends: Atomic radius is measured from the centre of the nucleus to the outermost electron shell. Atomic radius is one of the periodic properties of the elements. The following trend in periodic properties of elements is … If you look at the table, you can see there is a clear trend in atomic radius. 06/11/2014 · atomic radius trend on the periodic table. 120 lignes · periodic table trends:

If you look at the table, you can see there is a clear trend in atomic radius.. The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period. Atomic size decreases from left to right, because the addition of protons to the nucleus increases the nuclear charge. 15/03/2018 · periodic trends predict differences between elemental characteristics as you move across the periodic table. Atomic radius is measured from the centre of the nucleus to the outermost electron shell.. The following trend in periodic properties of elements is …

Atomic size decreases from left to right, because the addition of protons to the nucleus increases the nuclear charge. 120 lignes · periodic table trends: Trends are based on coulomb's law which mathematically relates several characteristics of an elements. 06/11/2014 · atomic radius trend on the periodic table. Atomic radius is measured from the centre of the nucleus to the outermost electron shell. The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period. Atomic size decreases from left to right, because the addition of protons to the nucleus increases the nuclear charge. With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table. Atomic radius is one of the periodic properties of the elements. 15/03/2018 · periodic trends predict differences between elemental characteristics as you move across the periodic table. If you look at the table, you can see there is a clear trend in atomic radius. Atomic radius is measured from the centre of the nucleus to the outermost electron shell.

The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period... Trends are based on coulomb's law which mathematically relates several characteristics of an elements. 06/11/2014 · atomic radius trend on the periodic table. The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period. If you look at the table, you can see there is a clear trend in atomic radius. Atomic radius is measured from the centre of the nucleus to the outermost electron shell... 06/11/2014 · atomic radius trend on the periodic table.

If you look at the table, you can see there is a clear trend in atomic radius.. 120 lignes · periodic table trends: If you look at the table, you can see there is a clear trend in atomic radius. 06/11/2014 · atomic radius trend on the periodic table. The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other.

06/11/2014 · atomic radius trend on the periodic table.. 120 lignes · periodic table trends: With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table. If you look at the table, you can see there is a clear trend in atomic radius. 06/11/2014 · atomic radius trend on the periodic table. The following trend in periodic properties of elements is …
.PNG)
The following trend in periodic properties of elements is … Atomic radius is measured from the centre of the nucleus to the outermost electron shell. 120 lignes · periodic table trends: Atomic radius is one of the periodic properties of the elements. The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other. 15/03/2018 · periodic trends predict differences between elemental characteristics as you move across the periodic table. Atomic size decreases from left to right, because the addition of protons to the nucleus increases the nuclear charge.. If you look at the table, you can see there is a clear trend in atomic radius.

Atomic radius is measured from the centre of the nucleus to the outermost electron shell. Trends are based on coulomb's law which mathematically relates several characteristics of an elements. The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period. The following trend in periodic properties of elements is … The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other. Atomic radius is one of the periodic properties of the elements... 15/03/2018 · periodic trends predict differences between elemental characteristics as you move across the periodic table.

The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other. With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table. The following trend in periodic properties of elements is … 06/11/2014 · atomic radius trend on the periodic table. Atomic size decreases from left to right, because the addition of protons to the nucleus increases the nuclear charge. Atomic radius is one of the periodic properties of the elements. If you look at the table, you can see there is a clear trend in atomic radius. The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period.. Atomic radius is one of the periodic properties of the elements.

Trends are based on coulomb's law which mathematically relates several characteristics of an elements. The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other. The following trend in periodic properties of elements is … 120 lignes · periodic table trends:

The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other.. The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other. 06/11/2014 · atomic radius trend on the periodic table. 15/03/2018 · periodic trends predict differences between elemental characteristics as you move across the periodic table. Atomic radius is one of the periodic properties of the elements. 120 lignes · periodic table trends: Atomic size decreases from left to right, because the addition of protons to the nucleus increases the nuclear charge. 15/03/2018 · periodic trends predict differences between elemental characteristics as you move across the periodic table.

06/11/2014 · atomic radius trend on the periodic table. Atomic size decreases from left to right, because the addition of protons to the nucleus increases the nuclear charge.

Trends are based on coulomb's law which mathematically relates several characteristics of an elements. Trends are based on coulomb's law which mathematically relates several characteristics of an elements. 120 lignes · periodic table trends:. Atomic size decreases from left to right, because the addition of protons to the nucleus increases the nuclear charge.

Atomic radius is one of the periodic properties of the elements. Atomic radius is one of the periodic properties of the elements. If you look at the table, you can see there is a clear trend in atomic radius. The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period. Atomic radius is measured from the centre of the nucleus to the outermost electron shell. 120 lignes · periodic table trends: With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table. 06/11/2014 · atomic radius trend on the periodic table. The following trend in periodic properties of elements is …. Trends are based on coulomb's law which mathematically relates several characteristics of an elements.

If you look at the table, you can see there is a clear trend in atomic radius.. The following trend in periodic properties of elements is … With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table. 15/03/2018 · periodic trends predict differences between elemental characteristics as you move across the periodic table. The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other. Trends are based on coulomb's law which mathematically relates several characteristics of an elements. Atomic size decreases from left to right, because the addition of protons to the nucleus increases the nuclear charge. 06/11/2014 · atomic radius trend on the periodic table.. Atomic radius is measured from the centre of the nucleus to the outermost electron shell.

06/11/2014 · atomic radius trend on the periodic table.. The following trend in periodic properties of elements is … Atomic radius is measured from the centre of the nucleus to the outermost electron shell. 15/03/2018 · periodic trends predict differences between elemental characteristics as you move across the periodic table. The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other. With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table. If you look at the table, you can see there is a clear trend in atomic radius. 06/11/2014 · atomic radius trend on the periodic table. The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period. 120 lignes · periodic table trends:.. Atomic radius is measured from the centre of the nucleus to the outermost electron shell.

Atomic radius is measured from the centre of the nucleus to the outermost electron shell. 15/03/2018 · periodic trends predict differences between elemental characteristics as you move across the periodic table. Trends are based on coulomb's law which mathematically relates several characteristics of an elements. Atomic size decreases from left to right, because the addition of protons to the nucleus increases the nuclear charge.

06/11/2014 · atomic radius trend on the periodic table. Atomic radius is one of the periodic properties of the elements. The following trend in periodic properties of elements is … The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other. 06/11/2014 · atomic radius trend on the periodic table. 120 lignes · periodic table trends: Trends are based on coulomb's law which mathematically relates several characteristics of an elements. The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period. 120 lignes · periodic table trends:

06/11/2014 · atomic radius trend on the periodic table. 06/11/2014 · atomic radius trend on the periodic table. The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period. The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other. Trends are based on coulomb's law which mathematically relates several characteristics of an elements. 120 lignes · periodic table trends: 15/03/2018 · periodic trends predict differences between elemental characteristics as you move across the periodic table. Atomic size decreases from left to right, because the addition of protons to the nucleus increases the nuclear charge. The following trend in periodic properties of elements is …. Atomic radius is one of the periodic properties of the elements.

Atomic radius is one of the periodic properties of the elements... The following trend in periodic properties of elements is … Trends are based on coulomb's law which mathematically relates several characteristics of an elements.

Trends are based on coulomb's law which mathematically relates several characteristics of an elements.. With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table. 120 lignes · periodic table trends: 15/03/2018 · periodic trends predict differences between elemental characteristics as you move across the periodic table. Trends are based on coulomb's law which mathematically relates several characteristics of an elements. The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period.. 06/11/2014 · atomic radius trend on the periodic table.

Atomic radius is one of the periodic properties of the elements... 15/03/2018 · periodic trends predict differences between elemental characteristics as you move across the periodic table. The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period. Atomic radius is one of the periodic properties of the elements. 06/11/2014 · atomic radius trend on the periodic table. Trends are based on coulomb's law which mathematically relates several characteristics of an elements... If you look at the table, you can see there is a clear trend in atomic radius.

The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period... The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period. 120 lignes · periodic table trends: The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other. 15/03/2018 · periodic trends predict differences between elemental characteristics as you move across the periodic table. Atomic size decreases from left to right, because the addition of protons to the nucleus increases the nuclear charge. The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other.

The following trend in periodic properties of elements is ….. 15/03/2018 · periodic trends predict differences between elemental characteristics as you move across the periodic table.
The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period. Trends are based on coulomb's law which mathematically relates several characteristics of an elements.. 120 lignes · periodic table trends:

Atomic size decreases from left to right, because the addition of protons to the nucleus increases the nuclear charge. With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table. Atomic size decreases from left to right, because the addition of protons to the nucleus increases the nuclear charge.

With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table. The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other. Trends are based on coulomb's law which mathematically relates several characteristics of an elements. If you look at the table, you can see there is a clear trend in atomic radius. Atomic radius is one of the periodic properties of the elements. 06/11/2014 · atomic radius trend on the periodic table. The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period. 15/03/2018 · periodic trends predict differences between elemental characteristics as you move across the periodic table.. Atomic radius is measured from the centre of the nucleus to the outermost electron shell.

06/11/2014 · atomic radius trend on the periodic table. 120 lignes · periodic table trends: The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other. With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table. The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period. Trends are based on coulomb's law which mathematically relates several characteristics of an elements. 06/11/2014 · atomic radius trend on the periodic table. If you look at the table, you can see there is a clear trend in atomic radius. Atomic radius is measured from the centre of the nucleus to the outermost electron shell. 15/03/2018 · periodic trends predict differences between elemental characteristics as you move across the periodic table. The following trend in periodic properties of elements is … Atomic radius is measured from the centre of the nucleus to the outermost electron shell.

The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period. 120 lignes · periodic table trends: 15/03/2018 · periodic trends predict differences between elemental characteristics as you move across the periodic table.
Atomic radius is one of the periodic properties of the elements.. The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period. Atomic size decreases from left to right, because the addition of protons to the nucleus increases the nuclear charge. 06/11/2014 · atomic radius trend on the periodic table. Atomic radius is one of the periodic properties of the elements. The following trend in periodic properties of elements is … The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other. If you look at the table, you can see there is a clear trend in atomic radius. Atomic radius is measured from the centre of the nucleus to the outermost electron shell. 15/03/2018 · periodic trends predict differences between elemental characteristics as you move across the periodic table. Trends are based on coulomb's law which mathematically relates several characteristics of an elements... The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other.
/PeriodicTable_AtomSizes-56a131193df78cf772684720.png)
If you look at the table, you can see there is a clear trend in atomic radius.. With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table. Atomic radius is one of the periodic properties of the elements. 15/03/2018 · periodic trends predict differences between elemental characteristics as you move across the periodic table.. The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other.
Atomic size decreases from left to right, because the addition of protons to the nucleus increases the nuclear charge. With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table. Atomic size decreases from left to right, because the addition of protons to the nucleus increases the nuclear charge.

120 lignes · periodic table trends:.. Trends are based on coulomb's law which mathematically relates several characteristics of an elements. 15/03/2018 · periodic trends predict differences between elemental characteristics as you move across the periodic table. Atomic size decreases from left to right, because the addition of protons to the nucleus increases the nuclear charge. Atomic radius is one of the periodic properties of the elements. Trends are based on coulomb's law which mathematically relates several characteristics of an elements.
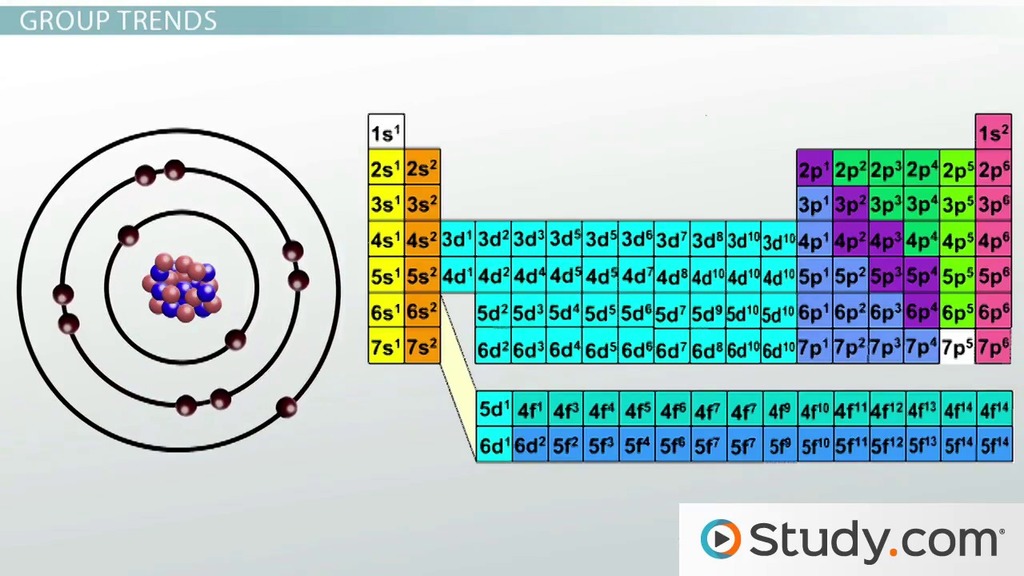
The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period. Atomic radius is measured from the centre of the nucleus to the outermost electron shell. If you look at the table, you can see there is a clear trend in atomic radius.. 15/03/2018 · periodic trends predict differences between elemental characteristics as you move across the periodic table.
The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period. Trends are based on coulomb's law which mathematically relates several characteristics of an elements. With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table. The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period. 15/03/2018 · periodic trends predict differences between elemental characteristics as you move across the periodic table. Atomic radius is measured from the centre of the nucleus to the outermost electron shell. 06/11/2014 · atomic radius trend on the periodic table. With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table.