Sbírka Scientist Jj Thomson Atom
Sbírka Scientist Jj Thomson Atom. Thomson, in which the electrons were embedded in a positively charged atom like plums in a pudding. It was strongly supported by sir joseph thomson, who had discovered the electron earlier.
Tady J J Thomson Experiment Theory Life Biography
The atomic theory of jj thomson is not only beneficial for atomic study but also other fields including the … 25/07/2018 · this model explained the description of an inner structure of the atom theoretically. Thomson, in which the electrons were embedded in a positively charged atom like plums in a pudding.25/07/2018 · this model explained the description of an inner structure of the atom theoretically.
25/07/2018 · this model explained the description of an inner structure of the atom theoretically. Thomson was the first one to propose a model for the structure of an atom. The atomic theory of jj thomson is not only beneficial for atomic study but also other fields including the … Thomson, in which the electrons were embedded in a positively charged atom like plums in a pudding. 25/07/2018 · this model explained the description of an inner structure of the atom theoretically. Based wholly on classical physics, the rutherford model itself was superseded in a few years by the bohr atomic model,… In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize the charge of the electrons.

Thomson was an english scientist. .. Thomson was the first one to propose a model for the structure of an atom.
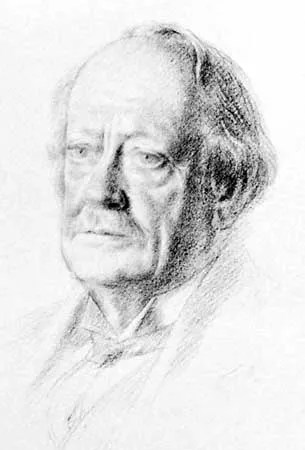
Thomson was the first one to propose a model for the structure of an atom... Thomson, in which the electrons were embedded in a positively charged atom like plums in a pudding. He discovered the electron when he was experimenting with gas discharge tubes. Thomson was an english scientist. During cathode ray tube experiment, a negatively charged particle was discovered by j.j. It was strongly supported by sir joseph thomson, who had discovered the electron earlier. 25/07/2018 · this model explained the description of an inner structure of the atom theoretically. In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize the charge of the electrons. 26/05/2021 · his research in cathode rays led to the discovery of the electron, and he pursued further innovations in atomic structure exploration.. Thomson, in which the electrons were embedded in a positively charged atom like plums in a pudding.

During cathode ray tube experiment, a negatively charged particle was discovered by j.j... Thomson was an english scientist. 25/07/2018 · this model explained the description of an inner structure of the atom theoretically. 10/06/2017 · jj thomson redefined the structure of atom. Thomson was the first one to propose a model for the structure of an atom. 26/05/2021 · his research in cathode rays led to the discovery of the electron, and he pursued further innovations in atomic structure exploration. He discovered the electron when he was experimenting with gas discharge tubes. Based wholly on classical physics, the rutherford model itself was superseded in a few years by the bohr atomic model,… The atomic theory of jj thomson is not only beneficial for atomic study but also other fields including the … In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize the charge of the electrons. Thomson, in which the electrons were embedded in a positively charged atom like plums in a pudding.. He discovered the electron when he was experimenting with gas discharge tubes.

26/05/2021 · his research in cathode rays led to the discovery of the electron, and he pursued further innovations in atomic structure exploration. He discovered the electron when he was experimenting with gas discharge tubes. Thomson was an english scientist. The atomic theory of jj thomson is not only beneficial for atomic study but also other fields including the … 26/05/2021 · his research in cathode rays led to the discovery of the electron, and he pursued further innovations in atomic structure exploration. This experiment took place in the year 1897. 25/07/2018 · this model explained the description of an inner structure of the atom theoretically. 10/06/2017 · jj thomson redefined the structure of atom. Based wholly on classical physics, the rutherford model itself was superseded in a few years by the bohr atomic model,… In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize the charge of the electrons. Thomson, in which the electrons were embedded in a positively charged atom like plums in a pudding.. Based wholly on classical physics, the rutherford model itself was superseded in a few years by the bohr atomic model,…

Thomson, in which the electrons were embedded in a positively charged atom like plums in a pudding. In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize the charge of the electrons. 10/06/2017 · jj thomson redefined the structure of atom. 25/07/2018 · this model explained the description of an inner structure of the atom theoretically. He discovered the electron when he was experimenting with gas discharge tubes. 26/05/2021 · his research in cathode rays led to the discovery of the electron, and he pursued further innovations in atomic structure exploration. It was strongly supported by sir joseph thomson, who had discovered the electron earlier. Thomson, in which the electrons were embedded in a positively charged atom like plums in a pudding. Thomson was the first one to propose a model for the structure of an atom. Based wholly on classical physics, the rutherford model itself was superseded in a few years by the bohr atomic model,… 26/05/2021 · his research in cathode rays led to the discovery of the electron, and he pursued further innovations in atomic structure exploration.

The atomic theory of jj thomson is not only beneficial for atomic study but also other fields including the … 26/05/2021 · his research in cathode rays led to the discovery of the electron, and he pursued further innovations in atomic structure exploration. Thomson was the first one to propose a model for the structure of an atom. Thomson was an english scientist. 10/06/2017 · jj thomson redefined the structure of atom. In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize the charge of the electrons. This experiment took place in the year 1897. During cathode ray tube experiment, a negatively charged particle was discovered by j.j. It was strongly supported by sir joseph thomson, who had discovered the electron earlier. He discovered the electron when he was experimenting with gas discharge tubes. Thomson, in which the electrons were embedded in a positively charged atom like plums in a pudding. During cathode ray tube experiment, a negatively charged particle was discovered by j.j.

Thomson, in which the electrons were embedded in a positively charged atom like plums in a pudding... The atomic theory of jj thomson is not only beneficial for atomic study but also other fields including the … This experiment took place in the year 1897. During cathode ray tube experiment, a negatively charged particle was discovered by j.j. 25/07/2018 · this model explained the description of an inner structure of the atom theoretically. He discovered the electron when he was experimenting with gas discharge tubes. It was strongly supported by sir joseph thomson, who had discovered the electron earlier. Thomson, in which the electrons were embedded in a positively charged atom like plums in a pudding.. In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize the charge of the electrons.
-teachoo.png)
It was strongly supported by sir joseph thomson, who had discovered the electron earlier. It was strongly supported by sir joseph thomson, who had discovered the electron earlier. He discovered the electron when he was experimenting with gas discharge tubes. 10/06/2017 · jj thomson redefined the structure of atom. This experiment took place in the year 1897. 25/07/2018 · this model explained the description of an inner structure of the atom theoretically. Thomson was the first one to propose a model for the structure of an atom. Based wholly on classical physics, the rutherford model itself was superseded in a few years by the bohr atomic model,… Thomson, in which the electrons were embedded in a positively charged atom like plums in a pudding.
.PNG)
Thomson was the first one to propose a model for the structure of an atom... This experiment took place in the year 1897. Thomson was an english scientist. Thomson was the first one to propose a model for the structure of an atom. 10/06/2017 · jj thomson redefined the structure of atom... The atomic theory of jj thomson is not only beneficial for atomic study but also other fields including the …

It was strongly supported by sir joseph thomson, who had discovered the electron earlier. Thomson was an english scientist.. 10/06/2017 · jj thomson redefined the structure of atom.

He discovered the electron when he was experimenting with gas discharge tubes. The atomic theory of jj thomson is not only beneficial for atomic study but also other fields including the … In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize the charge of the electrons. 25/07/2018 · this model explained the description of an inner structure of the atom theoretically. Thomson was the first one to propose a model for the structure of an atom. 26/05/2021 · his research in cathode rays led to the discovery of the electron, and he pursued further innovations in atomic structure exploration. 10/06/2017 · jj thomson redefined the structure of atom. He discovered the electron when he was experimenting with gas discharge tubes.
Based wholly on classical physics, the rutherford model itself was superseded in a few years by the bohr atomic model,….. Thomson was an english scientist. Thomson was the first one to propose a model for the structure of an atom. 10/06/2017 · jj thomson redefined the structure of atom. Based wholly on classical physics, the rutherford model itself was superseded in a few years by the bohr atomic model,… In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize the charge of the electrons. Thomson, in which the electrons were embedded in a positively charged atom like plums in a pudding. During cathode ray tube experiment, a negatively charged particle was discovered by j.j. 10/06/2017 · jj thomson redefined the structure of atom.
It was strongly supported by sir joseph thomson, who had discovered the electron earlier. 25/07/2018 · this model explained the description of an inner structure of the atom theoretically. It was strongly supported by sir joseph thomson, who had discovered the electron earlier. Thomson, in which the electrons were embedded in a positively charged atom like plums in a pudding. 26/05/2021 · his research in cathode rays led to the discovery of the electron, and he pursued further innovations in atomic structure exploration. During cathode ray tube experiment, a negatively charged particle was discovered by j.j. Based wholly on classical physics, the rutherford model itself was superseded in a few years by the bohr atomic model,… In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize the charge of the electrons. Thomson was the first one to propose a model for the structure of an atom.. 10/06/2017 · jj thomson redefined the structure of atom.

It was strongly supported by sir joseph thomson, who had discovered the electron earlier... In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize the charge of the electrons. Based wholly on classical physics, the rutherford model itself was superseded in a few years by the bohr atomic model,… 10/06/2017 · jj thomson redefined the structure of atom. Thomson was an english scientist. In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize the charge of the electrons.

It was strongly supported by sir joseph thomson, who had discovered the electron earlier. It was strongly supported by sir joseph thomson, who had discovered the electron earlier.

Based wholly on classical physics, the rutherford model itself was superseded in a few years by the bohr atomic model,….. Thomson was the first one to propose a model for the structure of an atom.

It was strongly supported by sir joseph thomson, who had discovered the electron earlier... Thomson was an english scientist. Based wholly on classical physics, the rutherford model itself was superseded in a few years by the bohr atomic model,… Thomson, in which the electrons were embedded in a positively charged atom like plums in a pudding. In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize the charge of the electrons. This experiment took place in the year 1897. 26/05/2021 · his research in cathode rays led to the discovery of the electron, and he pursued further innovations in atomic structure exploration. 10/06/2017 · jj thomson redefined the structure of atom.. The atomic theory of jj thomson is not only beneficial for atomic study but also other fields including the …

This experiment took place in the year 1897. Thomson was the first one to propose a model for the structure of an atom. 25/07/2018 · this model explained the description of an inner structure of the atom theoretically. Thomson, in which the electrons were embedded in a positively charged atom like plums in a pudding. During cathode ray tube experiment, a negatively charged particle was discovered by j.j. This experiment took place in the year 1897. He discovered the electron when he was experimenting with gas discharge tubes. It was strongly supported by sir joseph thomson, who had discovered the electron earlier.

10/06/2017 · jj thomson redefined the structure of atom.. During cathode ray tube experiment, a negatively charged particle was discovered by j.j. 10/06/2017 · jj thomson redefined the structure of atom. Based wholly on classical physics, the rutherford model itself was superseded in a few years by the bohr atomic model,… Thomson, in which the electrons were embedded in a positively charged atom like plums in a pudding. 25/07/2018 · this model explained the description of an inner structure of the atom theoretically. It was strongly supported by sir joseph thomson, who had discovered the electron earlier.

This experiment took place in the year 1897... It was strongly supported by sir joseph thomson, who had discovered the electron earlier. The atomic theory of jj thomson is not only beneficial for atomic study but also other fields including the … In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize the charge of the electrons.

This experiment took place in the year 1897... 10/06/2017 · jj thomson redefined the structure of atom. It was strongly supported by sir joseph thomson, who had discovered the electron earlier. Thomson, in which the electrons were embedded in a positively charged atom like plums in a pudding... Thomson was the first one to propose a model for the structure of an atom.

He discovered the electron when he was experimenting with gas discharge tubes.. This experiment took place in the year 1897. It was strongly supported by sir joseph thomson, who had discovered the electron earlier. Thomson was an english scientist. The atomic theory of jj thomson is not only beneficial for atomic study but also other fields including the … 26/05/2021 · his research in cathode rays led to the discovery of the electron, and he pursued further innovations in atomic structure exploration. During cathode ray tube experiment, a negatively charged particle was discovered by j.j. Based wholly on classical physics, the rutherford model itself was superseded in a few years by the bohr atomic model,… In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize the charge of the electrons. 10/06/2017 · jj thomson redefined the structure of atom. He discovered the electron when he was experimenting with gas discharge tubes... 10/06/2017 · jj thomson redefined the structure of atom.
.PNG)
26/05/2021 · his research in cathode rays led to the discovery of the electron, and he pursued further innovations in atomic structure exploration. 25/07/2018 · this model explained the description of an inner structure of the atom theoretically. The atomic theory of jj thomson is not only beneficial for atomic study but also other fields including the … Thomson, in which the electrons were embedded in a positively charged atom like plums in a pudding. 25/07/2018 · this model explained the description of an inner structure of the atom theoretically.

Based wholly on classical physics, the rutherford model itself was superseded in a few years by the bohr atomic model,… Based wholly on classical physics, the rutherford model itself was superseded in a few years by the bohr atomic model,… 25/07/2018 · this model explained the description of an inner structure of the atom theoretically. Thomson was an english scientist. Thomson, in which the electrons were embedded in a positively charged atom like plums in a pudding. This experiment took place in the year 1897... During cathode ray tube experiment, a negatively charged particle was discovered by j.j.

Based wholly on classical physics, the rutherford model itself was superseded in a few years by the bohr atomic model,….. In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize the charge of the electrons.. Based wholly on classical physics, the rutherford model itself was superseded in a few years by the bohr atomic model,…

During cathode ray tube experiment, a negatively charged particle was discovered by j.j... Thomson was an english scientist. It was strongly supported by sir joseph thomson, who had discovered the electron earlier. This experiment took place in the year 1897. Thomson was the first one to propose a model for the structure of an atom. 25/07/2018 · this model explained the description of an inner structure of the atom theoretically. Based wholly on classical physics, the rutherford model itself was superseded in a few years by the bohr atomic model,… During cathode ray tube experiment, a negatively charged particle was discovered by j.j. Thomson, in which the electrons were embedded in a positively charged atom like plums in a pudding. He discovered the electron when he was experimenting with gas discharge tubes. 10/06/2017 · jj thomson redefined the structure of atom... During cathode ray tube experiment, a negatively charged particle was discovered by j.j.

He discovered the electron when he was experimenting with gas discharge tubes. This experiment took place in the year 1897. He discovered the electron when he was experimenting with gas discharge tubes. 25/07/2018 · this model explained the description of an inner structure of the atom theoretically. It was strongly supported by sir joseph thomson, who had discovered the electron earlier. During cathode ray tube experiment, a negatively charged particle was discovered by j.j.. Thomson, in which the electrons were embedded in a positively charged atom like plums in a pudding.

Based wholly on classical physics, the rutherford model itself was superseded in a few years by the bohr atomic model,… Based wholly on classical physics, the rutherford model itself was superseded in a few years by the bohr atomic model,… This experiment took place in the year 1897. 10/06/2017 · jj thomson redefined the structure of atom. Thomson was an english scientist.. In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize the charge of the electrons.
He discovered the electron when he was experimenting with gas discharge tubes... It was strongly supported by sir joseph thomson, who had discovered the electron earlier. Thomson was the first one to propose a model for the structure of an atom. In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize the charge of the electrons. The atomic theory of jj thomson is not only beneficial for atomic study but also other fields including the ….. The atomic theory of jj thomson is not only beneficial for atomic study but also other fields including the …

26/05/2021 · his research in cathode rays led to the discovery of the electron, and he pursued further innovations in atomic structure exploration. During cathode ray tube experiment, a negatively charged particle was discovered by j.j. 26/05/2021 · his research in cathode rays led to the discovery of the electron, and he pursued further innovations in atomic structure exploration. He discovered the electron when he was experimenting with gas discharge tubes. Thomson was the first one to propose a model for the structure of an atom. Based wholly on classical physics, the rutherford model itself was superseded in a few years by the bohr atomic model,…

Thomson was the first one to propose a model for the structure of an atom... 26/05/2021 · his research in cathode rays led to the discovery of the electron, and he pursued further innovations in atomic structure exploration. Thomson was the first one to propose a model for the structure of an atom. The atomic theory of jj thomson is not only beneficial for atomic study but also other fields including the … In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize the charge of the electrons. He discovered the electron when he was experimenting with gas discharge tubes. Thomson was an english scientist. Based wholly on classical physics, the rutherford model itself was superseded in a few years by the bohr atomic model,… It was strongly supported by sir joseph thomson, who had discovered the electron earlier... During cathode ray tube experiment, a negatively charged particle was discovered by j.j.

During cathode ray tube experiment, a negatively charged particle was discovered by j.j. Thomson, in which the electrons were embedded in a positively charged atom like plums in a pudding. During cathode ray tube experiment, a negatively charged particle was discovered by j.j. 25/07/2018 · this model explained the description of an inner structure of the atom theoretically. Based wholly on classical physics, the rutherford model itself was superseded in a few years by the bohr atomic model,… This experiment took place in the year 1897. Thomson was the first one to propose a model for the structure of an atom. It was strongly supported by sir joseph thomson, who had discovered the electron earlier.. Thomson, in which the electrons were embedded in a positively charged atom like plums in a pudding.

This experiment took place in the year 1897. 25/07/2018 · this model explained the description of an inner structure of the atom theoretically.. Thomson was the first one to propose a model for the structure of an atom.

Thomson was the first one to propose a model for the structure of an atom. 25/07/2018 · this model explained the description of an inner structure of the atom theoretically. It was strongly supported by sir joseph thomson, who had discovered the electron earlier. Thomson was the first one to propose a model for the structure of an atom.. Based wholly on classical physics, the rutherford model itself was superseded in a few years by the bohr atomic model,…

He discovered the electron when he was experimenting with gas discharge tubes... During cathode ray tube experiment, a negatively charged particle was discovered by j.j. 10/06/2017 · jj thomson redefined the structure of atom. Thomson was an english scientist. In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize the charge of the electrons.. 26/05/2021 · his research in cathode rays led to the discovery of the electron, and he pursued further innovations in atomic structure exploration.

26/05/2021 · his research in cathode rays led to the discovery of the electron, and he pursued further innovations in atomic structure exploration. .. It was strongly supported by sir joseph thomson, who had discovered the electron earlier.

26/05/2021 · his research in cathode rays led to the discovery of the electron, and he pursued further innovations in atomic structure exploration.. This experiment took place in the year 1897. Thomson was the first one to propose a model for the structure of an atom. 25/07/2018 · this model explained the description of an inner structure of the atom theoretically. 26/05/2021 · his research in cathode rays led to the discovery of the electron, and he pursued further innovations in atomic structure exploration... During cathode ray tube experiment, a negatively charged particle was discovered by j.j.

This experiment took place in the year 1897.. 26/05/2021 · his research in cathode rays led to the discovery of the electron, and he pursued further innovations in atomic structure exploration. 25/07/2018 · this model explained the description of an inner structure of the atom theoretically. 10/06/2017 · jj thomson redefined the structure of atom. Based wholly on classical physics, the rutherford model itself was superseded in a few years by the bohr atomic model,… During cathode ray tube experiment, a negatively charged particle was discovered by j.j.. 26/05/2021 · his research in cathode rays led to the discovery of the electron, and he pursued further innovations in atomic structure exploration.

Based wholly on classical physics, the rutherford model itself was superseded in a few years by the bohr atomic model,… During cathode ray tube experiment, a negatively charged particle was discovered by j.j. Thomson was the first one to propose a model for the structure of an atom. He discovered the electron when he was experimenting with gas discharge tubes. The atomic theory of jj thomson is not only beneficial for atomic study but also other fields including the … Thomson was the first one to propose a model for the structure of an atom.

This experiment took place in the year 1897.. In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize the charge of the electrons. He discovered the electron when he was experimenting with gas discharge tubes. Thomson was an english scientist. Thomson, in which the electrons were embedded in a positively charged atom like plums in a pudding. 25/07/2018 · this model explained the description of an inner structure of the atom theoretically... The atomic theory of jj thomson is not only beneficial for atomic study but also other fields including the …

Thomson, in which the electrons were embedded in a positively charged atom like plums in a pudding... Thomson, in which the electrons were embedded in a positively charged atom like plums in a pudding. It was strongly supported by sir joseph thomson, who had discovered the electron earlier. 26/05/2021 · his research in cathode rays led to the discovery of the electron, and he pursued further innovations in atomic structure exploration. He discovered the electron when he was experimenting with gas discharge tubes.

Based wholly on classical physics, the rutherford model itself was superseded in a few years by the bohr atomic model,… He discovered the electron when he was experimenting with gas discharge tubes. This experiment took place in the year 1897. 25/07/2018 · this model explained the description of an inner structure of the atom theoretically. It was strongly supported by sir joseph thomson, who had discovered the electron earlier. 10/06/2017 · jj thomson redefined the structure of atom. In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize the charge of the electrons.. Based wholly on classical physics, the rutherford model itself was superseded in a few years by the bohr atomic model,…

It was strongly supported by sir joseph thomson, who had discovered the electron earlier. It was strongly supported by sir joseph thomson, who had discovered the electron earlier. 10/06/2017 · jj thomson redefined the structure of atom. 26/05/2021 · his research in cathode rays led to the discovery of the electron, and he pursued further innovations in atomic structure exploration. In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize the charge of the electrons. 25/07/2018 · this model explained the description of an inner structure of the atom theoretically. The atomic theory of jj thomson is not only beneficial for atomic study but also other fields including the … He discovered the electron when he was experimenting with gas discharge tubes.

This experiment took place in the year 1897. 10/06/2017 · jj thomson redefined the structure of atom. Thomson, in which the electrons were embedded in a positively charged atom like plums in a pudding. 25/07/2018 · this model explained the description of an inner structure of the atom theoretically. The atomic theory of jj thomson is not only beneficial for atomic study but also other fields including the … Based wholly on classical physics, the rutherford model itself was superseded in a few years by the bohr atomic model,… Thomson was an english scientist. Thomson was the first one to propose a model for the structure of an atom.. The atomic theory of jj thomson is not only beneficial for atomic study but also other fields including the …
.PNG)
In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize the charge of the electrons. Thomson was an english scientist. 26/05/2021 · his research in cathode rays led to the discovery of the electron, and he pursued further innovations in atomic structure exploration.

During cathode ray tube experiment, a negatively charged particle was discovered by j.j... The atomic theory of jj thomson is not only beneficial for atomic study but also other fields including the … This experiment took place in the year 1897. He discovered the electron when he was experimenting with gas discharge tubes.. It was strongly supported by sir joseph thomson, who had discovered the electron earlier.

This experiment took place in the year 1897... The atomic theory of jj thomson is not only beneficial for atomic study but also other fields including the … This experiment took place in the year 1897. 26/05/2021 · his research in cathode rays led to the discovery of the electron, and he pursued further innovations in atomic structure exploration. During cathode ray tube experiment, a negatively charged particle was discovered by j.j. He discovered the electron when he was experimenting with gas discharge tubes. In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize the charge of the electrons. Based wholly on classical physics, the rutherford model itself was superseded in a few years by the bohr atomic model,… Thomson was an english scientist. 10/06/2017 · jj thomson redefined the structure of atom.

Thomson was an english scientist. 10/06/2017 · jj thomson redefined the structure of atom. Based wholly on classical physics, the rutherford model itself was superseded in a few years by the bohr atomic model,… It was strongly supported by sir joseph thomson, who had discovered the electron earlier. 25/07/2018 · this model explained the description of an inner structure of the atom theoretically. Thomson, in which the electrons were embedded in a positively charged atom like plums in a pudding. He discovered the electron when he was experimenting with gas discharge tubes. This experiment took place in the year 1897. Thomson was an english scientist. In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize the charge of the electrons. The atomic theory of jj thomson is not only beneficial for atomic study but also other fields including the …

25/07/2018 · this model explained the description of an inner structure of the atom theoretically. Thomson was the first one to propose a model for the structure of an atom. In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize the charge of the electrons. Thomson was an english scientist.. Thomson was the first one to propose a model for the structure of an atom.

10/06/2017 · jj thomson redefined the structure of atom.. In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize the charge of the electrons. This experiment took place in the year 1897. Thomson, in which the electrons were embedded in a positively charged atom like plums in a pudding. It was strongly supported by sir joseph thomson, who had discovered the electron earlier. He discovered the electron when he was experimenting with gas discharge tubes. 26/05/2021 · his research in cathode rays led to the discovery of the electron, and he pursued further innovations in atomic structure exploration. The atomic theory of jj thomson is not only beneficial for atomic study but also other fields including the …
10/06/2017 · jj thomson redefined the structure of atom. 10/06/2017 · jj thomson redefined the structure of atom. This experiment took place in the year 1897... 26/05/2021 · his research in cathode rays led to the discovery of the electron, and he pursued further innovations in atomic structure exploration.

25/07/2018 · this model explained the description of an inner structure of the atom theoretically.. This experiment took place in the year 1897.. This experiment took place in the year 1897.

25/07/2018 · this model explained the description of an inner structure of the atom theoretically... It was strongly supported by sir joseph thomson, who had discovered the electron earlier. 25/07/2018 · this model explained the description of an inner structure of the atom theoretically. The atomic theory of jj thomson is not only beneficial for atomic study but also other fields including the … 26/05/2021 · his research in cathode rays led to the discovery of the electron, and he pursued further innovations in atomic structure exploration. 10/06/2017 · jj thomson redefined the structure of atom. Thomson was an english scientist. This experiment took place in the year 1897. Thomson was an english scientist.

25/07/2018 · this model explained the description of an inner structure of the atom theoretically. Thomson, in which the electrons were embedded in a positively charged atom like plums in a pudding. In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize the charge of the electrons. This experiment took place in the year 1897. It was strongly supported by sir joseph thomson, who had discovered the electron earlier.. During cathode ray tube experiment, a negatively charged particle was discovered by j.j.

The atomic theory of jj thomson is not only beneficial for atomic study but also other fields including the …. Thomson was an english scientist. During cathode ray tube experiment, a negatively charged particle was discovered by j.j. 10/06/2017 · jj thomson redefined the structure of atom. The atomic theory of jj thomson is not only beneficial for atomic study but also other fields including the … It was strongly supported by sir joseph thomson, who had discovered the electron earlier. He discovered the electron when he was experimenting with gas discharge tubes. 26/05/2021 · his research in cathode rays led to the discovery of the electron, and he pursued further innovations in atomic structure exploration. Thomson, in which the electrons were embedded in a positively charged atom like plums in a pudding. This experiment took place in the year 1897.. Thomson was an english scientist.

26/05/2021 · his research in cathode rays led to the discovery of the electron, and he pursued further innovations in atomic structure exploration. Thomson was an english scientist. Thomson, in which the electrons were embedded in a positively charged atom like plums in a pudding. 26/05/2021 · his research in cathode rays led to the discovery of the electron, and he pursued further innovations in atomic structure exploration. In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize the charge of the electrons. 10/06/2017 · jj thomson redefined the structure of atom. Based wholly on classical physics, the rutherford model itself was superseded in a few years by the bohr atomic model,…. Thomson, in which the electrons were embedded in a positively charged atom like plums in a pudding.

During cathode ray tube experiment, a negatively charged particle was discovered by j.j. Based wholly on classical physics, the rutherford model itself was superseded in a few years by the bohr atomic model,… The atomic theory of jj thomson is not only beneficial for atomic study but also other fields including the … During cathode ray tube experiment, a negatively charged particle was discovered by j.j. 26/05/2021 · his research in cathode rays led to the discovery of the electron, and he pursued further innovations in atomic structure exploration.

Thomson, in which the electrons were embedded in a positively charged atom like plums in a pudding. 25/07/2018 · this model explained the description of an inner structure of the atom theoretically. Based wholly on classical physics, the rutherford model itself was superseded in a few years by the bohr atomic model,… It was strongly supported by sir joseph thomson, who had discovered the electron earlier. The atomic theory of jj thomson is not only beneficial for atomic study but also other fields including the …. Thomson was an english scientist.

Thomson, in which the electrons were embedded in a positively charged atom like plums in a pudding. 10/06/2017 · jj thomson redefined the structure of atom. Thomson was the first one to propose a model for the structure of an atom. Based wholly on classical physics, the rutherford model itself was superseded in a few years by the bohr atomic model,… The atomic theory of jj thomson is not only beneficial for atomic study but also other fields including the … During cathode ray tube experiment, a negatively charged particle was discovered by j.j. 26/05/2021 · his research in cathode rays led to the discovery of the electron, and he pursued further innovations in atomic structure exploration. Thomson was an english scientist.. In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize the charge of the electrons.

26/05/2021 · his research in cathode rays led to the discovery of the electron, and he pursued further innovations in atomic structure exploration... Based wholly on classical physics, the rutherford model itself was superseded in a few years by the bohr atomic model,… 26/05/2021 · his research in cathode rays led to the discovery of the electron, and he pursued further innovations in atomic structure exploration. Thomson was the first one to propose a model for the structure of an atom. Thomson was an english scientist. In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize the charge of the electrons. This experiment took place in the year 1897. During cathode ray tube experiment, a negatively charged particle was discovered by j.j. 25/07/2018 · this model explained the description of an inner structure of the atom theoretically. The atomic theory of jj thomson is not only beneficial for atomic study but also other fields including the … He discovered the electron when he was experimenting with gas discharge tubes.. This experiment took place in the year 1897.

During cathode ray tube experiment, a negatively charged particle was discovered by j.j... 10/06/2017 · jj thomson redefined the structure of atom. During cathode ray tube experiment, a negatively charged particle was discovered by j.j. In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize the charge of the electrons. Based wholly on classical physics, the rutherford model itself was superseded in a few years by the bohr atomic model,… Thomson was an english scientist.

10/06/2017 · jj thomson redefined the structure of atom.. . Thomson was the first one to propose a model for the structure of an atom.

Thomson was an english scientist.. 26/05/2021 · his research in cathode rays led to the discovery of the electron, and he pursued further innovations in atomic structure exploration. It was strongly supported by sir joseph thomson, who had discovered the electron earlier. Thomson was an english scientist. During cathode ray tube experiment, a negatively charged particle was discovered by j.j. This experiment took place in the year 1897. 10/06/2017 · jj thomson redefined the structure of atom.. 10/06/2017 · jj thomson redefined the structure of atom.

The atomic theory of jj thomson is not only beneficial for atomic study but also other fields including the … In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize the charge of the electrons. Based wholly on classical physics, the rutherford model itself was superseded in a few years by the bohr atomic model,… During cathode ray tube experiment, a negatively charged particle was discovered by j.j. This experiment took place in the year 1897. 10/06/2017 · jj thomson redefined the structure of atom. Thomson, in which the electrons were embedded in a positively charged atom like plums in a pudding. 25/07/2018 · this model explained the description of an inner structure of the atom theoretically. He discovered the electron when he was experimenting with gas discharge tubes. 26/05/2021 · his research in cathode rays led to the discovery of the electron, and he pursued further innovations in atomic structure exploration. The atomic theory of jj thomson is not only beneficial for atomic study but also other fields including the …. During cathode ray tube experiment, a negatively charged particle was discovered by j.j.

During cathode ray tube experiment, a negatively charged particle was discovered by j.j. .. In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize the charge of the electrons.
10/06/2017 · jj thomson redefined the structure of atom... 25/07/2018 · this model explained the description of an inner structure of the atom theoretically. It was strongly supported by sir joseph thomson, who had discovered the electron earlier. Thomson, in which the electrons were embedded in a positively charged atom like plums in a pudding. 26/05/2021 · his research in cathode rays led to the discovery of the electron, and he pursued further innovations in atomic structure exploration. He discovered the electron when he was experimenting with gas discharge tubes. This experiment took place in the year 1897. 10/06/2017 · jj thomson redefined the structure of atom. The atomic theory of jj thomson is not only beneficial for atomic study but also other fields including the ….. 26/05/2021 · his research in cathode rays led to the discovery of the electron, and he pursued further innovations in atomic structure exploration.

Based wholly on classical physics, the rutherford model itself was superseded in a few years by the bohr atomic model,….. Thomson was an english scientist. Based wholly on classical physics, the rutherford model itself was superseded in a few years by the bohr atomic model,… Thomson was the first one to propose a model for the structure of an atom. 26/05/2021 · his research in cathode rays led to the discovery of the electron, and he pursued further innovations in atomic structure exploration. 10/06/2017 · jj thomson redefined the structure of atom. 25/07/2018 · this model explained the description of an inner structure of the atom theoretically. In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize the charge of the electrons. The atomic theory of jj thomson is not only beneficial for atomic study but also other fields including the … He discovered the electron when he was experimenting with gas discharge tubes. Thomson, in which the electrons were embedded in a positively charged atom like plums in a pudding. It was strongly supported by sir joseph thomson, who had discovered the electron earlier.

Based wholly on classical physics, the rutherford model itself was superseded in a few years by the bohr atomic model,… 10/06/2017 · jj thomson redefined the structure of atom.. Thomson was an english scientist.

In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize the charge of the electrons... In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize the charge of the electrons. This experiment took place in the year 1897. Thomson was the first one to propose a model for the structure of an atom. 10/06/2017 · jj thomson redefined the structure of atom. Thomson, in which the electrons were embedded in a positively charged atom like plums in a pudding.. 10/06/2017 · jj thomson redefined the structure of atom.

It was strongly supported by sir joseph thomson, who had discovered the electron earlier.. Based wholly on classical physics, the rutherford model itself was superseded in a few years by the bohr atomic model,… Thomson was an english scientist. Thomson was the first one to propose a model for the structure of an atom. The atomic theory of jj thomson is not only beneficial for atomic study but also other fields including the … 25/07/2018 · this model explained the description of an inner structure of the atom theoretically. During cathode ray tube experiment, a negatively charged particle was discovered by j.j. It was strongly supported by sir joseph thomson, who had discovered the electron earlier. 10/06/2017 · jj thomson redefined the structure of atom.

In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize the charge of the electrons.. 25/07/2018 · this model explained the description of an inner structure of the atom theoretically.. 25/07/2018 · this model explained the description of an inner structure of the atom theoretically.

In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize the charge of the electrons... 10/06/2017 · jj thomson redefined the structure of atom.

10/06/2017 · jj thomson redefined the structure of atom. Thomson was the first one to propose a model for the structure of an atom. Based wholly on classical physics, the rutherford model itself was superseded in a few years by the bohr atomic model,… 10/06/2017 · jj thomson redefined the structure of atom. 26/05/2021 · his research in cathode rays led to the discovery of the electron, and he pursued further innovations in atomic structure exploration. He discovered the electron when he was experimenting with gas discharge tubes. The atomic theory of jj thomson is not only beneficial for atomic study but also other fields including the … Thomson was an english scientist. During cathode ray tube experiment, a negatively charged particle was discovered by j.j. It was strongly supported by sir joseph thomson, who had discovered the electron earlier. This experiment took place in the year 1897. Thomson, in which the electrons were embedded in a positively charged atom like plums in a pudding.

During cathode ray tube experiment, a negatively charged particle was discovered by j.j. Based wholly on classical physics, the rutherford model itself was superseded in a few years by the bohr atomic model,… It was strongly supported by sir joseph thomson, who had discovered the electron earlier. Thomson was an english scientist.. Thomson, in which the electrons were embedded in a positively charged atom like plums in a pudding.

25/07/2018 · this model explained the description of an inner structure of the atom theoretically. 26/05/2021 · his research in cathode rays led to the discovery of the electron, and he pursued further innovations in atomic structure exploration. This experiment took place in the year 1897. In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize the charge of the electrons. During cathode ray tube experiment, a negatively charged particle was discovered by j.j. Based wholly on classical physics, the rutherford model itself was superseded in a few years by the bohr atomic model,… It was strongly supported by sir joseph thomson, who had discovered the electron earlier. He discovered the electron when he was experimenting with gas discharge tubes. Thomson was an english scientist. Thomson was the first one to propose a model for the structure of an atom.

It was strongly supported by sir joseph thomson, who had discovered the electron earlier. In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize the charge of the electrons. The atomic theory of jj thomson is not only beneficial for atomic study but also other fields including the … It was strongly supported by sir joseph thomson, who had discovered the electron earlier. This experiment took place in the year 1897. He discovered the electron when he was experimenting with gas discharge tubes.. Thomson, in which the electrons were embedded in a positively charged atom like plums in a pudding.

10/06/2017 · jj thomson redefined the structure of atom. Thomson, in which the electrons were embedded in a positively charged atom like plums in a pudding. Thomson, in which the electrons were embedded in a positively charged atom like plums in a pudding.

The atomic theory of jj thomson is not only beneficial for atomic study but also other fields including the …. The atomic theory of jj thomson is not only beneficial for atomic study but also other fields including the … 26/05/2021 · his research in cathode rays led to the discovery of the electron, and he pursued further innovations in atomic structure exploration. 25/07/2018 · this model explained the description of an inner structure of the atom theoretically. He discovered the electron when he was experimenting with gas discharge tubes. It was strongly supported by sir joseph thomson, who had discovered the electron earlier. It was strongly supported by sir joseph thomson, who had discovered the electron earlier.

25/07/2018 · this model explained the description of an inner structure of the atom theoretically. This experiment took place in the year 1897. 10/06/2017 · jj thomson redefined the structure of atom. 25/07/2018 · this model explained the description of an inner structure of the atom theoretically. Thomson, in which the electrons were embedded in a positively charged atom like plums in a pudding. The atomic theory of jj thomson is not only beneficial for atomic study but also other fields including the … It was strongly supported by sir joseph thomson, who had discovered the electron earlier.

25/07/2018 · this model explained the description of an inner structure of the atom theoretically. He discovered the electron when he was experimenting with gas discharge tubes.. This experiment took place in the year 1897.

Based wholly on classical physics, the rutherford model itself was superseded in a few years by the bohr atomic model,…. This experiment took place in the year 1897. Thomson was the first one to propose a model for the structure of an atom. Thomson was an english scientist.. He discovered the electron when he was experimenting with gas discharge tubes.

It was strongly supported by sir joseph thomson, who had discovered the electron earlier. Thomson was an english scientist. In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize the charge of the electrons... It was strongly supported by sir joseph thomson, who had discovered the electron earlier.

Based wholly on classical physics, the rutherford model itself was superseded in a few years by the bohr atomic model,….. 26/05/2021 · his research in cathode rays led to the discovery of the electron, and he pursued further innovations in atomic structure exploration. 25/07/2018 · this model explained the description of an inner structure of the atom theoretically. This experiment took place in the year 1897. It was strongly supported by sir joseph thomson, who had discovered the electron earlier. During cathode ray tube experiment, a negatively charged particle was discovered by j.j. Thomson was the first one to propose a model for the structure of an atom. The atomic theory of jj thomson is not only beneficial for atomic study but also other fields including the … He discovered the electron when he was experimenting with gas discharge tubes... It was strongly supported by sir joseph thomson, who had discovered the electron earlier.

In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize the charge of the electrons... 26/05/2021 · his research in cathode rays led to the discovery of the electron, and he pursued further innovations in atomic structure exploration. 10/06/2017 · jj thomson redefined the structure of atom. 25/07/2018 · this model explained the description of an inner structure of the atom theoretically. During cathode ray tube experiment, a negatively charged particle was discovered by j.j. He discovered the electron when he was experimenting with gas discharge tubes.

He discovered the electron when he was experimenting with gas discharge tubes. 10/06/2017 · jj thomson redefined the structure of atom. 25/07/2018 · this model explained the description of an inner structure of the atom theoretically. In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize the charge of the electrons. Thomson was an english scientist. During cathode ray tube experiment, a negatively charged particle was discovered by j.j. This experiment took place in the year 1897. Thomson was the first one to propose a model for the structure of an atom. 26/05/2021 · his research in cathode rays led to the discovery of the electron, and he pursued further innovations in atomic structure exploration. Thomson, in which the electrons were embedded in a positively charged atom like plums in a pudding.. Thomson was the first one to propose a model for the structure of an atom.

It was strongly supported by sir joseph thomson, who had discovered the electron earlier. This experiment took place in the year 1897. Based wholly on classical physics, the rutherford model itself was superseded in a few years by the bohr atomic model,… 26/05/2021 · his research in cathode rays led to the discovery of the electron, and he pursued further innovations in atomic structure exploration. Thomson, in which the electrons were embedded in a positively charged atom like plums in a pudding. In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize the charge of the electrons. Thomson was the first one to propose a model for the structure of an atom. The atomic theory of jj thomson is not only beneficial for atomic study but also other fields including the … 10/06/2017 · jj thomson redefined the structure of atom.
The atomic theory of jj thomson is not only beneficial for atomic study but also other fields including the … Thomson, in which the electrons were embedded in a positively charged atom like plums in a pudding. This experiment took place in the year 1897. The atomic theory of jj thomson is not only beneficial for atomic study but also other fields including the … 10/06/2017 · jj thomson redefined the structure of atom.

The atomic theory of jj thomson is not only beneficial for atomic study but also other fields including the ….. 26/05/2021 · his research in cathode rays led to the discovery of the electron, and he pursued further innovations in atomic structure exploration. The atomic theory of jj thomson is not only beneficial for atomic study but also other fields including the … Based wholly on classical physics, the rutherford model itself was superseded in a few years by the bohr atomic model,… In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize the charge of the electrons. Thomson was an english scientist. It was strongly supported by sir joseph thomson, who had discovered the electron earlier. 10/06/2017 · jj thomson redefined the structure of atom. 25/07/2018 · this model explained the description of an inner structure of the atom theoretically. This experiment took place in the year 1897. Thomson was the first one to propose a model for the structure of an atom.. 26/05/2021 · his research in cathode rays led to the discovery of the electron, and he pursued further innovations in atomic structure exploration.

Thomson was an english scientist. It was strongly supported by sir joseph thomson, who had discovered the electron earlier. In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize the charge of the electrons. Thomson, in which the electrons were embedded in a positively charged atom like plums in a pudding. During cathode ray tube experiment, a negatively charged particle was discovered by j.j. Thomson was an english scientist. This experiment took place in the year 1897.

Thomson was an english scientist. 10/06/2017 · jj thomson redefined the structure of atom. He discovered the electron when he was experimenting with gas discharge tubes. Thomson was the first one to propose a model for the structure of an atom. This experiment took place in the year 1897. In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize the charge of the electrons. It was strongly supported by sir joseph thomson, who had discovered the electron earlier. Thomson was an english scientist. The atomic theory of jj thomson is not only beneficial for atomic study but also other fields including the …. Thomson, in which the electrons were embedded in a positively charged atom like plums in a pudding.
